Streptococcus is a genus of gram-positive coccus or spherical bacteria that belongs to the family Streptococcaceae, within the order Lactobacillales, in the phylum Bacillota. Cell division in streptococci occurs along a single axis, so as they grow, they tend to form pairs or chains that may appear bent or twisted. This differs from staphylococci, which divide along multiple axes, thereby generating irregular, grape-like clusters of cells. Most streptococci are oxidase-negative and catalase-negative, and many are facultative anaerobes.

Streptococcus pyogenes is a species of Gram-positive, aerotolerant bacteria in the genus Streptococcus. These bacteria are extracellular, and made up of non-motile and non-sporing cocci that tend to link in chains. They are clinically important for humans, as they are an infrequent, but usually pathogenic, part of the skin microbiota that can cause Group A streptococcal infection. S. pyogenes is the predominant species harboring the Lancefield group A antigen, and is often called group A Streptococcus (GAS). However, both Streptococcus dysgalactiae and the Streptococcus anginosus group can possess group A antigen as well. Group A streptococci, when grown on blood agar, typically produce small (2–3 mm) zones of beta-hemolysis, a complete destruction of red blood cells. The name group A (beta-hemolytic) Streptococcus is thus also used.
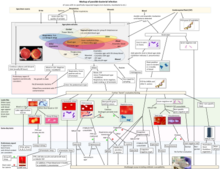
Scarlet fever, also known as scarlatina, is an infectious disease caused by Streptococcus pyogenes, a Group A streptococcus (GAS). It most commonly affects children between five and 15 years of age. The signs and symptoms include a sore throat, fever, headache, swollen lymph nodes, and a characteristic rash. The face is flushed and the rash is red and blanching. It typically feels like sandpaper and the tongue may be red and bumpy. The rash occurs as a result of capillary damage by exotoxins produced by S.pyogenes. On darker-pigmented skin the rash may be hard to discern.

Streptococcal pharyngitis, also known as streptococcal sore throat, is pharyngitis caused by Streptococcus pyogenes, a gram-positive, group A streptococcus. Common symptoms include fever, sore throat, red tonsils, and enlarged lymph nodes in the front of the neck. A headache and nausea or vomiting may also occur. Some develop a sandpaper-like rash which is known as scarlet fever. Symptoms typically begin one to three days after exposure and last seven to ten days.
Pharyngitis is inflammation of the back of the throat, known as the pharynx. It typically results in a sore throat and fever. Other symptoms may include a runny nose, cough, headache, difficulty swallowing, swollen lymph nodes, and a hoarse voice. Symptoms usually last 3–5 days, but can be longer depending on cause. Complications can include sinusitis and acute otitis media. Pharyngitis is a type of upper respiratory tract infection.

Sore throat, also known as throat pain, is pain or irritation of the throat. Usually, causes of sore throat include:

Rheumatic fever (RF) is an inflammatory disease that can involve the heart, joints, skin, and brain. The disease typically develops two to four weeks after a streptococcal throat infection. Signs and symptoms include fever, multiple painful joints, involuntary muscle movements, and occasionally a characteristic non-itchy rash known as erythema marginatum. The heart is involved in about half of the cases. Damage to the heart valves, known as rheumatic heart disease (RHD), usually occurs after repeated attacks but can sometimes occur after one. The damaged valves may result in heart failure, atrial fibrillation and infection of the valves.

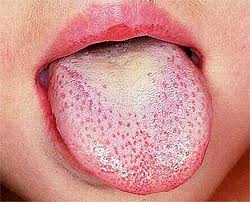
Tonsillitis is inflammation of the tonsils in the upper part of the throat. It can be acute or chronic. Acute tonsillitis typically has a rapid onset. Symptoms may include sore throat, fever, enlargement of the tonsils, trouble swallowing, and enlarged lymph nodes around the neck. Complications include peritonsillar abscess (Quinsy).

Erythema marginatum is an acquired skin condition which primarily affects the arms, trunk, and legs. It is a type of erythema characterised by bright pink or red circular lesions which have sharply-defined borders and faint central clearing. The lesions typically range from 3 to 10 cm in size, and are distributed symmetrically over the torso and inner surfaces of the limbs and extensor surfaces. The lesions last between one and four weeks but have been known to be present on patients for as long as several months.
A complication in medicine, or medical complication, is an unfavorable result of a disease, health condition, or treatment. Complications may adversely affect the prognosis, or outcome, of a disease. Complications generally involve a worsening in the severity of the disease or the development of new signs, symptoms, or pathological changes that may become widespread throughout the body and affect other organ systems. Thus, complications may lead to the development of new diseases resulting from previously existing diseases. Complications may also arise as a result of various treatments.

Streptococcus agalactiae is a gram-positive coccus with a tendency to form chains. It is a beta-hemolytic, catalase-negative, and facultative anaerobe.

Group B streptococcal infection, also known as Group B streptococcal disease or just Group B strep infection, is the infectious disease caused by the bacterium Streptococcus agalactiae, which is the most common human pathogen belonging to the group B of the Lancefield classification of streptococci—hence the group B stretococcal (GBS) infection nomenclature. Infection with GBS can cause serious illness and sometimes death, especially in newborns, the elderly, and people with compromised immune systems. The most severe form of group B streptococcal disease is neonatal meningitis in infants, which is frequently lethal and can cause permanent neuro-cognitive impairment.
Anti-streptolysin O is the antibody made against streptolysin O, an immunogenic, oxygen-labile streptococcal hemolytic exotoxin produced by most strains of group A and many strains of groups C and G Streptococcus bacteria. The "O" in the name stands for oxygen-labile; the other related toxin being oxygen-stable streptolysin-S. The main function of streptolysin O is to cause hemolysis —in particular, beta-hemolysis.
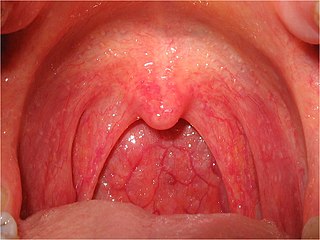
The rapid strep test (RST) is a rapid antigen detection test (RADT) that is widely used in clinics to assist in the diagnosis of bacterial pharyngitis caused by group A streptococci (GAS), sometimes termed strep throat. There are currently several types of rapid strep test in use, each employing a distinct technology. However, they all work by detecting the presence of GAS in the throat of a person by responding to GAS-specific antigens on a throat swab.

Streptococcus dysgalactiae is a gram positive, beta-haemolytic, coccal bacterium belonging to the family Streptococcaceae. It is capable of infecting both humans and animals, but is most frequently encountered as a commensal of the alimentary tract, genital tract, or less commonly, as a part of the skin flora. The clinical manifestations in human disease range from superficial skin-infections and tonsillitis, to severe necrotising fasciitis and bacteraemia. The incidence of invasive disease has been reported to be rising. Several different animal species are susceptible to infection by S. dysgalactiae, but bovine mastitis and infectious arthritis in lambs have been most frequently reported.

Streptococcal intertrigo is a skin condition that is secondary to a streptococcal bacterial infection. It is often seen in infants and young children and can be characterized by a fiery-red color of the skin, foul odor with an absence of satellite lesions, and skin softening in the neck, armpits or folds of the groin. Newborn children and infants commonly develop intertrigo because of physical features such as deep skin folds, short neck, and flexed posture. Prompt diagnosis by a medical professional and treatment with topical and/or oral antibiotics can effectively relieve symptoms.
Perianal cellulitis, also known as perianitis or perianal streptococcal dermatitis, is a bacterial infection affecting the lower layers of the skin (cellulitis) around the anus. It presents as bright redness in the skin and can be accompanied by pain, difficulty defecating, itching, and bleeding. This disease is considered a complicated skin and soft tissue infection (cSSTI) because of the involvement of the deeper soft tissues.
Bacteriophage T12 is a bacteriophage that infects Streptococcus pyogenes bacteria. It is a proposed species of the family Siphoviridae in the order Caudovirales also known as tailed viruses. It converts a harmless strain of bacteria into a virulent strain. It carries the speA gene which codes for erythrogenic toxin A. speA is also known as streptococcal pyogenic exotoxin A, scarlet fever toxin A, or even scarlatinal toxin. Note that the name of the gene "speA" is italicized; the name of the toxin "speA" is not italicized. Erythrogenic toxin A converts a harmless, non-virulent strain of Streptococcus pyogenes to a virulent strain through lysogeny, a life cycle which is characterized by the ability of the genome to become a part of the host cell and be stably maintained there for generations. Phages with a lysogenic life cycle are also called temperate phages. Bacteriophage T12, proposed member of family Siphoviridae including related speA-carrying bacteriophages, is also a prototypic phage for all the speA-carrying phages of Streptococcus pyogenes, meaning that its genome is the prototype for the genomes of all such phages of S. pyogenes. It is the main suspect as the cause of scarlet fever, an infectious disease that affects small children.

In late 2022, an ongoing disease outbreak caused by the bacterium Streptococcus pyogenes, a Lancefield group A streptococcus, began in the United Kingdom. It is often referred to as the Strep A outbreak in the media. These bacteria cause group A streptococcal infections and scarlet fever. In the UK, 516 deaths from iGAS have been recorded, of which 61 were children, 52 in England, five in Wales, three in Scotland, and one in Northern Ireland.
Streptococcosis are diseases varying from mild to fatal side effects of the infection which originate from the bacterial group streptococcus. Some of the areas of infection include wounds, body tissue, and respiratory areas. Research within horses, dogs, cats, wound injuries and swine infections have been done to document specific side effects from streptococcosis. Streptococcosis can occur to both humans and animals, the most common including horses, guinea pigs, dogs, cats and fish; while uncommon animals infected include monkeys, cattle, sheep, goats, ferrets and poultry. The wide range of diseases is due to the variability of streptococcus strains which thus creates multiple species for the diseases to occur.