Cymene describes organic compounds with the formula CH3C6H4CH(CH3)2. Three isomers exist: 1,2- 1,3-, and 1,4-. All are colorless liquids, immiscible in water, with similar boiling points. They are classified as aromatic hydrocarbons. They bear two substituents: an isopropyl (CH(CH3)2) group and a methyl group. [1]
| Cymenes | |||
| Name | o-Cymene | m-Cymene | p-Cymene |
|---|---|---|---|
| Structural formula |  |  | 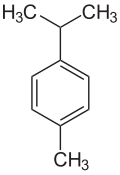 |
| CAS number | 527-84-4 | 535-77-3 | 99-87-6 |
| melting point (°C) | −71.54 | −63.75 | −67.94 |
| boiling point (°C) | 178.15 | 175.05 | 177.10 |