The centimetre–gram–second system of units is a variant of the metric system based on the centimetre as the unit of length, the gram as the unit of mass, and the second as the unit of time. All CGS mechanical units are unambiguously derived from these three base units, but there are several different ways in which the CGS system was extended to cover electromagnetism.

The kilogram is the base unit of mass in the International System of Units (SI), having the unit symbol kg. 'Kilogram' means 'one thousand grams' and is colloquially abbreviated to kilo.

The litre or liter is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metres (m3). A cubic decimetre occupies a volume of 10 cm × 10 cm × 10 cm and is thus equal to one-thousandth of a cubic metre.

The International System of Units, internationally known by the abbreviation SI, is the modern form of the metric system and the world's most widely used system of measurement. It is the only system of measurement with official status in nearly every country in the world, employed in science, technology, industry, and everyday commerce. The SI system is coordinated by the International Bureau of Weights and Measures which is abbreviated BIPM from French: Bureau international des poids et mesures.

The SI base units are the standard units of measurement defined by the International System of Units (SI) for the seven base quantities of what is now known as the International System of Quantities: they are notably a basic set from which all other SI units can be derived. The units and their physical quantities are the second for time, the metre for length or distance, the kilogram for mass, the ampere for electric current, the kelvin for thermodynamic temperature, the mole for amount of substance, and the candela for luminous intensity. The SI base units are a fundamental part of modern metrology, and thus part of the foundation of modern science and technology.

The second is a unit of time, historically defined as 1⁄86400 of a day – this factor derived from the division of the day first into 24 hours, then to 60 minutes and finally to 60 seconds each.
A metric prefix is a unit prefix that precedes a basic unit of measure to indicate a multiple or submultiple of the unit. All metric prefixes used today are decadic. Each prefix has a unique symbol that is prepended to any unit symbol. The prefix kilo-, for example, may be added to gram to indicate multiplication by one thousand: one kilogram is equal to one thousand grams. The prefix milli-, likewise, may be added to metre to indicate division by one thousand; one millimetre is equal to one thousandth of a metre.
SI derived units are units of measurement derived from the seven SI base units specified by the International System of Units (SI). They can be expressed as a product of one or more of the base units, possibly scaled by an appropriate power of exponentiation. Some are dimensionless, as when the units cancel out in ratios of like quantities. SI coherent derived units involve only a trivial proportionality factor, not requiring conversion factors.

The mole (symbol mol) is a unit of measurement, the base unit in the International System of Units (SI) for amount of substance, an SI base quantity proportional to the number of elementary entities of a substance. One mole is an aggregate of exactly 6.02214076×1023 elementary entities (approximately 602 sextillion or 602 billion times a trillion), which can be atoms, molecules, ions, ion pairs, or other particles. The number of particles in a mole is the Avogadro number (symbol N0) and the numerical value of the Avogadro constant (symbol NA) expressed in mol-1. The value was chosen on the basis of the historical definition of the mole as the amount of substance that corresponds to the number of atoms in 12 grams of 12C, which made the mass of a mole of a compound expressed in grams, numerically equal to the average molecular mass or formula mass of the compound expressed in daltons. With the 2019 revision of the SI, the numerical equivalence is now only approximate but may be assumed for all practical purposes.

The metric system is a system of measurement that standardizes a set of base units and a nomenclature for describing relatively large and small quantities via decimal-based multiplicative unit prefixes. Though the rules governing the metric system have changed over time, the modern definition, the International System of Units (SI), defines the metric prefixes and seven base units: metre (m), kilogram (kg), second (s), ampere (A), kelvin (K), mole (mol), and candela (cd).

Metric time is the measure of time intervals using the metric system. The modern SI system defines the second as the base unit of time, and forms multiples and submultiples with metric prefixes such as kiloseconds and milliseconds. Other units of time – minute, hour, and day – are accepted for use with SI, but are not part of it. Metric time is a measure of time intervals, while decimal time is a means of recording time of day.
A base unit of measurement is a unit of measurement adopted for a base quantity. A base quantity is one of a conventionally chosen subset of physical quantities, where no quantity in the subset can be expressed in terms of the others. The SI base units, or Systéme International d'unités, consists of the metre, kilogram, second, ampere, kelvin, mole and candela.
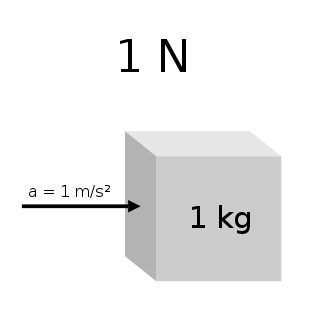
The newton is the unit of force in the International System of Units (SI). Expressed in terms of SI base units, it is 1 kg⋅m/s2, the force that accelerates a mass of one kilogram at one metre per second squared.
The ohm is the unit of electrical resistance in the International System of Units (SI). It is named after German physicist Georg Ohm. Various empirically derived standard units for electrical resistance were developed in connection with early telegraphy practice, and the British Association for the Advancement of Science proposed a unit derived from existing units of mass, length and time, and of a convenient scale for practical work as early as 1861.
The metre, kilogram, second system of units, also known more briefly as MKS units or the MKS system, is a physical system of measurement based on the metre, kilogram, and second (MKS) as base units. Distances are described in terms of metres, mass in terms of kilograms and time in seconds. Derived units are defined using the appropriate combinations, such as velocity in metres per second. Some units have their own names, such as the newton unit of force which is the combination kilogram metre per second squared.

In 2019, four of the seven SI base units specified in the International System of Quantities were redefined in terms of natural physical constants, rather than human artefacts such as the standard kilogram. Effective 20 May 2019, the 144th anniversary of the Metre Convention, the kilogram, ampere, kelvin, and mole are now defined by setting exact numerical values, when expressed in SI units, for the Planck constant, the elementary electric charge, the Boltzmann constant, and the Avogadro constant, respectively. The second, metre, and candela had previously been redefined using physical constants. The four new definitions aimed to improve the SI without changing the value of any units, ensuring continuity with existing measurements. In November 2018, the 26th General Conference on Weights and Measures (CGPM) unanimously approved these changes, which the International Committee for Weights and Measures (CIPM) had proposed earlier that year after determining that previously agreed conditions for the change had been met. These conditions were satisfied by a series of experiments that measured the constants to high accuracy relative to the old SI definitions, and were the culmination of decades of research.

The history of the metric system began during the Age of Enlightenment with measures of length and weight derived from nature, along with their decimal multiples and fractions. The system became the standard of France and Europe within half a century. Other measures with unity ratios were added, and the system went on to be adopted across the world.

The following outline is provided as an overview of and topical guide to the metric system:

A coherent system of units is a system of units of measurement used to express physical quantities that are defined in such a way that the equations relating the numerical values expressed in the units of the system have exactly the same form, including numerical factors, as the corresponding equations directly relating the quantities. It is a system in which every quantity has a unique unit, or one that does not use conversion factors.
Since its introduction in 1960, the base units for the International system of units, known as SI, have changed several times. Tables in this article summarize those changes.












