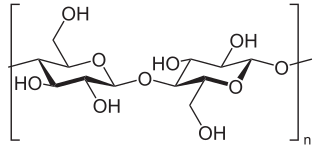
Cellulose is an organic compound with the formula (C
6H
10O
5)
n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most abundant organic polymer on Earth. The cellulose content of cotton fibre is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%.

Pulp is a fibrous lignocellulosic material prepared by chemically, semi-chemically or mechanically producing cellulosic fibers from wood, fiber crops, waste paper, or rags. Mixed with water and other chemicals or plant-based additives, pulp is the major raw material used in papermaking and the industrial production of other paper products.

Paper engineering is a branch of engineering that deals with the usage of physical science and life sciences in conjunction with mathematics as applied to the converting of raw materials into useful paper products and co-products. The field applies various principles in process engineering and unit operations to the manufacture of paper, chemicals, energy and related materials. The following timeline shows some of the key steps in the development of the science of chemical and bioprocess engineering:

Paperboard is a thick paper-based material. While there is no rigid differentiation between paper and paperboard, paperboard is generally thicker than paper and has certain superior attributes such as foldability and rigidity. According to ISO standards, paperboard is a paper with a grammage above 250 g/m2, but there are exceptions. Paperboard can be single- or multi-ply.

A paper machine is an industrial machine which is used in the pulp and paper industry to create paper in large quantities at high speed. Modern paper-making machines are based on the principles of the Fourdrinier Machine, which uses a moving woven mesh to create a continuous paper web by filtering out the fibres held in a paper stock and producing a continuously moving wet mat of fibre. This is dried in the machine to produce a strong paper web.
Sizing or size is a substance that is applied to, or incorporated into, other materials—especially papers and textiles—to act as a protective filler or glaze. Sizing is used in papermaking and textile manufacturing to change the absorption and wear characteristics of those materials.

The kraft process (also known as kraft pulping or sulfate process) is a process for conversion of wood into wood pulp, which consists of almost pure cellulose fibres, the main component of paper. The kraft process involves treatment of wood chips with a hot mixture of water, sodium hydroxide (NaOH), and sodium sulfide (Na2S), known as white liquor, that breaks the bonds that link lignin, hemicellulose, and cellulose. The technology entails several steps, both mechanical and chemical. It is the dominant method for producing paper. In some situations, the process has been controversial because kraft plants can release odorous products and in some situations produce substantial liquid wastes.
A binder or binding agent is any material or substance that holds or draws other materials together to form a cohesive whole mechanically, chemically, by adhesion or cohesion.

A pulp mill is a manufacturing facility that converts wood chips or other plant fiber sources into a thick fiber board which can be shipped to a paper mill for further processing. Pulp can be manufactured using mechanical, semi-chemical, or fully chemical methods. The finished product may be either bleached or non-bleached, depending on the customer requirements.

Laundry detergent is a type of detergent used for cleaning dirty laundry (clothes). Laundry detergent is manufactured in powder and liquid form.

Kraft paper or kraft is paper or paperboard (cardboard) produced from chemical pulp produced in the kraft process.

Coated paper is paper that has been coated by a mixture of materials or a polymer to impart certain qualities to the paper, including weight, surface gloss, smoothness, or reduced ink absorbency. Various materials, including kaolinite, calcium carbonate, bentonite, and talc, can be used to coat paper for high-quality printing used in the packaging industry and in magazines.

Inkjet paper is a special fine paper designed for inkjet printers, typically classified by its weight, brightness and smoothness, and sometimes by its opacity.
Bleaching of wood pulp is the chemical processing of wood pulp to lighten its color and whiten the pulp. The primary product of wood pulp is paper, for which whiteness is an important characteristic. These processes and chemistry are also applicable to the bleaching of non-wood pulps, such as those made from bamboo or kenaf.
The sulfite process produces wood pulp that is almost pure cellulose fibers by treating wood chips with solutions of sulfite and bisulfite ions. These chemicals cleave the bonds between the cellulose and lignin components of the lignocellulose. A variety of sulfite/bisulfite salts are used, including sodium (Na+), calcium (Ca2+), potassium (K+), magnesium (Mg2+), and ammonium (NH4+). The lignin is converted to lignosulfonates, which are soluble and can be separated from the cellulose fibers. For the production of cellulose, the sulfite process competes with the Kraft process which produces stronger fibers and is less environmentally costly.
Dissolving pulp, also called dissolving cellulose, is bleached wood pulp or cotton linters that has a high cellulose content. It has special properties including a high level of brightness and uniform molecular-weight distribution. This pulp is manufactured for uses that require a high chemical purity, and particularly low hemicellulose content, since the chemically similar hemicellulose can interfere with subsequent processes. Dissolving pulp is so named because it is not made into paper, but dissolved either in a solvent or by derivatization into a homogeneous solution, which makes it completely chemically accessible and removes any remaining fibrous structure. Once dissolved, it can be spun into textile fibers, or chemically reacted to produce derivatized celluloses, such cellulose triacetate, a plastic-like material formed into fibers or films, or cellulose ethers such as methyl cellulose, used as a thickener.

Nanocellulose is a term referring to a family of cellulosic materials that have at least one of their dimensions in the nanoscale. Examples of nanocellulosic materials are microfibrilated cellulose, cellulose nanofibers or cellulose nanocrystals. Nanocellulose may be obtained from natural cellulose fibers through a variety of production processes. This family of materials possesses interesting properties suitable for a wide range of potential applications.
Wet Processing Engineering is one of the major streams in Textile Engineering or Textile manufacturing which refers to the engineering of textile chemical processes and associated applied science. The other three streams in textile engineering are yarn engineering, fabric engineering, and apparel engineering. The processes of this stream are involved or carried out in an aqueous stage. Hence, it is called a wet process which usually covers pre-treatment, dyeing, printing, and finishing.
The surface chemistry of paper is responsible for many important paper properties, such as gloss, waterproofing, and printability. Many components are used in the paper-making process that affect the surface.

Alkenyl succinic anhydrides (ASA) are derivatives of succinic anhydrides. One H of the succinic anhydride ring is replaced with an iso-alkenyl chain (C14 to C22). ASA's are colorless and usually viscous liquids. They are widely used, especially in surface sizing of paper, paperboard, and cardboard, as well as in the hydrophobicization of cellulose fibers. Products treated with it show reduced penetration of aqueous media, such as inks or drinks (like milk or fruit juices).
















