Related Research Articles

Epithelium is one of the four basic types of animal tissue, along with connective tissue, muscle tissue and nervous tissue. It is a thin, continuous, protective layer of compactly packed cells with little intercellular matrix. Epithelial tissues line the outer surfaces of organs and blood vessels throughout the body, as well as the inner surfaces of cavities in many internal organs. An example is the epidermis, the outermost layer of the skin.

The blastocyst is a structure formed in the early development of mammals. It possesses an inner cell mass (ICM) which subsequently forms the embryo. The outer layer of the blastocyst consists of cells collectively called the trophoblast. This layer surrounds the inner cell mass and a fluid-filled cavity known as the blastocoel. The trophoblast gives rise to the chorion and amnion that surround the embryo. The placenta derives from the embryonic chorion and the underlying uterine tissue of the mother. The name "blastocyst" arises from the Greek βλαστός blastos and κύστις kystis. In other animals this is called a blastula.

The proximal tubule is the segment of the nephron in kidneys which begins from the renal pole of the Bowman's capsule to the beginning of loop of Henle. It can be further classified into the proximal convoluted tubule (PCT) and the proximal straight tubule (PST).
Cell junctions are a class of cellular structures consisting of multiprotein complexes that provide contact or adhesion between neighboring cells or between a cell and the extracellular matrix in animals. They also maintain the paracellular barrier of epithelia and control paracellular transport. Cell junctions are especially abundant in epithelial tissues. Combined with cell adhesion molecules and extracellular matrix, cell junctions help hold animal cells together.

Tight junctions, also known as occluding junctions or zonulae occludentes are multiprotein junctional complexes whose canonical function is to prevent leakage of solutes and water and seals the paracellular pathway. Tight junctions may also serve as leaky pathways by forming selective channels for small cations, anions, or water. Tight junctions are present mostly in vertebrates. The corresponding junctions that occur in invertebrates are septate junctions.
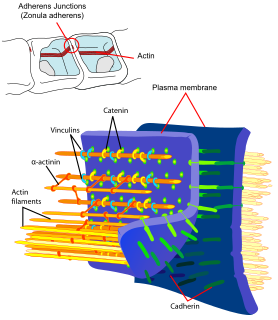
Adherens junctions are protein complexes that occur at cell–cell junctions, cell–matrix junctions in epithelial and endothelial tissues, usually more basal than tight junctions. An adherens junction is defined as a cell junction whose cytoplasmic face is linked to the actin cytoskeleton. They can appear as bands encircling the cell or as spots of attachment to the extracellular matrix . Adherens junctions uniquely disassemble in uterine epithelial cells to allow the blastocyst to penetrate between epithelial cells.

Claudins are a family of proteins which, along with occludin, are the most important components of the tight junctions. Tight junctions establish the paracellular barrier that controls the flow of molecules in the intercellular space between the cells of an epithelium. They have four transmembrane domains, with the N-terminus and the C-terminus in the cytoplasm.
Transcytosis is a type of transcellular transport in which various macromolecules are transported across the interior of a cell. Macromolecules are captured in vesicles on one side of the cell, drawn across the cell, and ejected on the other side. Examples of macromolecules transported include IgA, transferrin, and insulin. While transcytosis is most commonly observed in epithelial cells, the process is also present elsewhere. Blood capillaries are a well-known site for transcytosis, though it occurs in other cells, including neurons, osteoclasts and M cells of the intestine.

In humans, implantation is the stage of human reproduction at which the embryo adheres to the wall of the uterus. At this stage of prenatal development, the conceptus is called a blastocyst. Once this adhesion is successful, the female is considered to be pregnant and the embryo will receive oxygen and nutrients from the mother in order to grow.
Terminal bar is a histological term given to the unresolved group of junctional complexes that attach adjacent epithelial cells on their lateral surfaces: the Zonula Occludens, Zonula Adherens, Macula Adherens and Macula Communicans.

The intestinal epithelium is the single cell layer that form the luminal surface (lining) of both the small and large intestine (colon) of the gastrointestinal tract. Composed of simple columnar epithelial cells, it serves two main functions: absorbing useful substances into the body and restricting the entry of harmful substances. As part of its protective role, the intestinal epithelium forms an important component of the intestinal mucosal barrier. Certain diseases and conditions are caused by functional defects in the intestinal epithelium. On the other hand, various diseases and conditions can lead to its dysfunction which, in turn, can lead to further complications.
Involution is the shrinking or return of an organ to a former size. At a cellular level, involution is characterized by the process of proteolysis of the basement membrane, leading to epithelial regression and apoptosis, with accompanying stromal fibrosis. The consequent reduction in cell number and reorganization of stromal tissue leads to the reduction in the size of the organ.

Cingulin-like protein 1, also known as paracingulin or junction-associated-coiled-coil protein (JACOP), is a protein which is encoded by the CGNL1 gene.
Cell polarity is a fundamental feature of many types of cells. Epithelial cells are one example of a polarized cell type, featuring distinct 'apical', 'lateral' and 'basal' plasma membrane domains. Epithelial cells connect to one another via their lateral membranes to form epithelial sheets that line cavities and surfaces throughout the animal body. Each plasma membrane domain has a distinct protein composition, giving them distinct properties and allowing directional transport of molecules across the epithelial sheet. How epithelial cells generate and maintain polarity remains unclear, but certain molecules have been found to play a key role.
Cell–cell interaction refers to the direct interactions between cell surfaces that play a crucial role in the development and function of multicellular organisms. These interactions allow cells to communicate with each other in response to changes in their microenvironment. This ability to send and receive signals is essential for the survival of the cell. Interactions between cells can be stable such as those made through cell junctions. These junctions are involved in the communication and organization of cells within a particular tissue. Others are transient or temporary such as those between cells of the immune system or the interactions involved in tissue inflammation. These types of intercellular interactions are distinguished from other types such as those between cells and the extracellular matrix. The loss of communication between cells can result in uncontrollable cell growth and cancer.
Pinopodes are protrusions on the apical cellular membrane of uterine epithelial cells.
An intercellular cleft is a channel between two cells through which molecules may travel and gap junctions and tight junctions may be present. Most notably, intercellular clefts are often found between epithelial cells and the endothelium of blood vessels and lymphatic vessels, also helping to form the blood-nerve barrier surrounding nerves. Intercellular clefts are important for allowing the transportation of fluids and small solute matter through the endothelium.
The plasma membrane transformation is a concept introduced by Christopher R. Murphy of The University of Sydney to encapsulate the idea that a series of changes in the plasma membrane of uterine epithelial cells is essential to the development of the receptivity of the uterus (womb) for attachment of the blastocyst and the beginning of a pregnancy.

The vaginal epithelium is the inner lining of the vagina consisting of multiple layers of (squamous) cells. The basal membrane provides the support for the first layer of the epithelium-the basal layer. The intermediate layers lie upon the basal layer, and the superficial layer is the outermost layer of the epithelium. Anatomists have described the epithelium as consisting of as many as 40 distinct layers. The mucus found on the epithelium is secreted by the cervix and uterus. The rugae of the epithelium create an involuted surface and result in a large surface area that covers 360 cm2. This large surface area allows the trans-epithelial absorption of some medications via the vaginal route.
Tight junction proteins are molecules situated at the tight junctions of epithelial, endothelial and myelinated cells. This multiprotein junctional complex has a regulatory function in passage of ions, water and solutes through the paracellular pathway. It can also coordinate the motion of lipids and proteins between the apical and basolateral surfaces of the plasma membrane. Thereby tight junction conducts signaling molecules, that influence the differentiation, proliferation and polarity of cells. So tight junction plays a key role in maintenance of osmotic balance and trans-cellular transport of tissue specific molecules. Nowadays is known more than 40 different proteins, that are involved in these selective TJ channels.
References
- ↑ Finn, C; Porter, D (1975). The Uterus. London: Paul Elek (Scientific Books) Ltd.
- ↑ Bucci, M., & Murphy, C. R. (2001). Hormonal control of enzyme activity during the plasma membrane transformation of uterine epithelial cells. Cell biology international, 25, 859-871.
- ↑ Luxford, K. A., & Murphy, C. R. (1989). Cytoskeletal alterations in the microvilli of uterine epithelial cells during early pregnancy. Acta histochemica, 87(2), 131-136.
- ↑ Luxford, K. A., & Murphy, C. R. (1992). Changes in the apical microfilaments of rat uterine epithelial cells in response to estradiol and progesterone. The Anatomical Record, 233(4), 521-526.
- 1 2 3 4 5 Nicholson, M., Lindsay, L. A., & Murphy, C. R. (2010). Ovarian hormones control the changing expression of claudins and occludin in rat uterine epithelial cells during early pregnancy. Acta histochemica, 112(1), 42-52.
- ↑ Yurchenco, P. D., & Schittny, J. C. (1990). Molecular architecture of basement membranes. The FASEB Journal, 4(6), 1577-1590.
- ↑ Murphy, C. R., & Shaw, T. J. (1994). Plasma membrane transformation: a common response of uterine epithelial cells during the peri‐implantation period. Cell biology international, 18(12), 1115-1128.
- 1 2 SCHLAFKE, S., & ENDERS, A. C. (1975). Cellular basis of interaction between trophoblast and uterus at implantation. Biology of Reproduction, 12(1), 41-65.
- 1 2 Enders, A. C., & Schlafke, S. (1967). A morphological analysis of the early implantation stages in the rat. American Journal of Anatomy, 120(2), 185-225.
- ↑ HEWITT, K., BEER, A. E., & GRINNELL, F. (1979). Disappearance of anionic sites from the surface of the rat endometrial epithelium at the time of blastocyst implantation. Biology of reproduction, 21(3), 691-707.
- ↑ Murphy, C. R. (2001). The plasma membrane transformation: a key concept in uterine receptivity. Reproductive Medicine Review, 9(03), 197-208.
- 1 2 Murphy, C. R., Swift, J. G., Mukherjee, T. M., & Rogers, A. W. (1982). The structure of tight junctions between uterine luminal epithelial cells at different stages of pregnancy in the rat. Cell and Tissue Research, 223(2), 281-286.
- 1 2 3 4 Lindsay, L. A., & Murphy, C. R. (2004). Redistribution of aquaporins in uterine epithelial cells at the time of implantation in the rat. Acta histochemica, 106(4), 299-307.
- ↑ Murphy, C. R., Rogers, A. W., Swift, J. G., & Mukherjee, T. M. (1980). Ovarian hormones alter tight junction structure in uterine luminal epithelial cells. Micron (1969), 11(3), 375-376.
- ↑ Colegio, O. R., Van Itallie, C. M., McCrea, H. J., Rahner, C., & Anderson, J. M. (2002). Claudins create charge-selective channels in the paracellular pathway between epithelial cells. American Journal of Physiology. Cell Physiology, 283(1), C142-C147.
- 1 2 Enders, A. C., & Nelson, D. M. (1973). Pinocytotic activity of the uterus of the rat. American Journal of Anatomy, 138(3), 277-299.
- ↑ Lundkvist, Ö. (1979). Morphometric estimation of stromal edema during delayed implantation in the rat. Cell and Tissue Research, 199(2), 339-348.
- ↑ Kennedy, T. G., & Armstrong, D. T. (1975). Loss of uterine luminal fluid in the rat: relative importance of changing peripheral levels of estrogen and progesterone. Endocrinology, 97(6), 1379-1385.
- ↑ PARR, M. B., & PARR, E. L. (1974). Uterine luminal epithelium: protrusions mediate endocytosis, not apocrine secretion, in the rat. Biology of reproduction, 11(2), 220-233.
- ↑ Tsang, L. L., Chan, L. N., & Chan, H. C. (2004). Altered cyclic expression of epithelial Na+ channel subunits and cystic fibrosis transmembrane conductance regulator in mouse endometrium by a low sodium diet. Cell biology international, 28(7), 549-555.
- ↑ Salleh, N., Baines, D. L., Naftalin, R. J., & Milligan, S. R. (2005). The hormonal control of uterine luminal fluid secretion and absorption. The Journal of membrane biology, 206(1), 17-28.
- ↑ Tsang, L. L., Chan, L. N., Wang, X. F., So, S. C., Yuen, J. P., Fiscus, R. R., & Chan, H. C. (2001). Enhanced epithelial Na (+) channel (ENaC) activity in mouse endometrial epithelium by upregulation of gammaENaC subunit. The Japanese journal of physiology, 51(4), 539-543.
- ↑ Orchard, M. D., & Murphy, C. R. (2002). Alterations in tight junction molecules of uterine epithelial cells during early pregnancy in the rat. Acta histochemica, 104(2), 149-155.
- 1 2 Lindsay, L. A., & Murphy, C. R. (2006). Redistribution of aquaporins 1 and 5 in the rat uterus is dependent on progesterone: a study with light and electron microscopy. Reproduction, 131(2), 369-378.