
The endometrium is the inner epithelial layer, along with its mucous membrane, of the mammalian uterus. It has a basal layer and a functional layer: the basal layer contains stem cells which regenerate the functional layer. The functional layer thickens and then is shed during menstruation in humans and some other mammals, including apes, Old World monkeys, some species of bat, the elephant shrew and the Cairo spiny mouse. In most other mammals, the endometrium is reabsorbed in the estrous cycle. During pregnancy, the glands and blood vessels in the endometrium further increase in size and number. Vascular spaces fuse and become interconnected, forming the placenta, which supplies oxygen and nutrition to the embryo and fetus. The speculated presence of an endometrial microbiota has been argued against.

The uterus or womb is the main hormone-responsive, secondary sex organ of the female reproductive system in humans, and most other mammals. Events occurring within the uterus are described with the term in utero. In the human, the lower end of the uterus, the cervix, opens into the vagina, while the upper end, the fundus, is connected to the fallopian tubes. It is within the uterus that the embryo and later fetus develops during gestation. In the human embryo, the uterus develops from the paramesonephric ducts which fuse into the single organ known as a simplex uterus. The uterus has different forms in many other animals and in some it exists as two separate uteri known as a duplex uterus.

The menstrual cycle is a series of natural changes in hormone production and the structures of the uterus and ovaries of the female reproductive system that make pregnancy possible. The ovarian cycle controls the production and release of eggs and the cyclic release of estrogen and progesterone. The uterine cycle governs the preparation and maintenance of the lining of the uterus (womb) to receive an embryo. These cycles are concurrent and coordinated, normally last between 21 and 35 days in adult women, with a median length of 28 days, and continue for about 30–45 years.

The blastocyst is a structure formed in the early development of mammals. It possesses an inner cell mass (ICM) which subsequently forms the embryo. The outer layer of the blastocyst consists of cells collectively called the trophoblast. This layer surrounds the inner cell mass and a fluid-filled cavity known as the blastocoel. The trophoblast gives rise to the chorion and amnion that surround the embryo. The placenta derives from the embryonic chorion and the underlying uterine tissue of the mother. The name "blastocyst" arises from the Greek βλαστός blastos and κύστις kystis. In other animals this is a structure consisting of an undifferentiated ball of cells and is called a blastula.

Trophoblasts are cells that form the outer layer of a blastocyst. They are present four days after fertilization in humans. They provide nutrients to the embryo and develop into a large part of the placenta. They form during the first stage of pregnancy and are the first cells to differentiate from the fertilized egg to become extraembryonic structures and do not directly contribute to the embryo. After gastrulation, the trophoblast is contiguous with the ectoderm of the embryo and is referred to as the trophectoderm. After the first differentiation, the cells in the human embryo lose their totipotency and are no longer totipotent stem cells because they cannot form a trophoblast. They are now pluripotent stem cells.

The decidua is the modified mucosal lining of the uterus that forms in preparation for pregnancy. It is formed in a process called decidualization under the influence of progesterone. Endometrial cells become highly characteristic. The decidua forms the maternal part of the placenta and remains for the duration of the pregnancy. It is shed off during childbirth—hence why the term is used, "decidua" having the meaning of falling away, as in the word deciduous.

"Cytotrophoblast" is the name given to both the inner layer of the trophoblast or the cells that live there. It is interior to the syncytiotrophoblast and external to the wall of the blastocyst in a developing embryo.
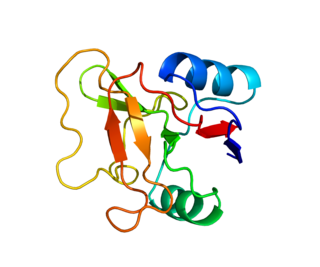
L-selectin, also known as CD62L, is a cell adhesion molecule found on the cell surface of leukocytes and the preimplantation embryo. It belongs to the selectin family of proteins, which recognize sialylated carbohydrate groups containing a Sialyl LewisX (sLeX) determinant. L-selectin plays an important role in both the innate and adaptive immune responses by facilitating leukocyte-endothelial cell adhesion events. These tethering interactions are essential for the trafficking of monocytes and neutrophils into inflamed tissue as well as the homing of lymphocytes to secondary lymphoid organs. L-selectin is also expressed by lymphoid primed hematopoietic stem cells and may participate in the migration of these stem cells to the primary lymphoid organs. In addition to its function in the immune response, L-selectin is expressed on embryonic cells and facilitates the attachment of the blastocyst to the endometrial endothelium during human embryo implantation.

Decidualization is a process that results in significant changes to cells of the endometrium in preparation for, and during, pregnancy. This includes morphological and functional changes to endometrial stromal cells (ESCs), the presence of decidual white blood cells (leukocytes), and vascular changes to maternal arteries. The sum of these changes results in the endometrium changing into a structure called the decidua. In humans, the decidua is shed during childbirth.
Before the fertilized ovum reaches the uterus, the mucous membrane of the body of the uterus undergoes important changes and is then known as the decidua. The thickness and vascularity of the mucous membrane are greatly increased; its glands are elongated and open on its free surface by funnel-shaped orifices, while their deeper portions are tortuous and dilated into irregular spaces. The interglandular tissue is also increased in quantity, and is crowded with large round, oval, or polygonal cells, termed decidual cells. Their enlargement is due to glycogen and lipid accumulation in the cytoplasm allowing these cells to provide a rich source of nutrition for the developing embryo. Decidual cells are also thought to control the invasion of the endometrium by trophoblast cells.

Human embryonic development, or human embryogenesis, is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilisation occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form a single cell called a zygote and the germinal stage of development commences. Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. Human embryology is the study of this development during the first eight weeks after fertilisation. The normal period of gestation (pregnancy) is about nine months or 40 weeks.

Oviduct-specific glycoprotein also known as oviductal glycoprotein (OGP) or estrogen-dependent oviduct protein (EGP) or mucin-9 (MUC9) is a protein that in humans is encoded by the OVGP1 gene.
Pinopodes are protrusions on the apical cellular membrane of uterine epithelial cells.
Hormonal regulation occurs at every stage of development. A milieu of hormones simultaneously affects development of the fetus during embryogenesis and the mother, including human chorionic gonadotropin (hCG) and progesterone (P4).
Menstruation is the shedding of the uterine lining (endometrium). It occurs on a regular basis in uninseminated sexually reproductive-age females of certain mammal species.
The plasma membrane transformation is a concept introduced by Christopher R. Murphy of The University of Sydney to encapsulate the idea that a series of changes in the plasma membrane of uterine epithelial cells is essential to the development of the receptivity of the uterus (womb) for attachment of the blastocyst and the beginning of a pregnancy.
The internal surface of the uterus is lined by uterine epithelial cells which undergo dramatic changes during pregnancy. The role of the uterine epithelial cells is to selectively allow the blastocyst to implant at a specific time. All other times of the cycle, these uterine epithelial cells are refractory to blastocyst implantation. Uterine epithelial cells have a similar structure in most species and the changes which occur in the uterine epithelial cells at the time of blastocyst implantation are also conserved among most species.
Repeated Implantation failure (RIF) is the failure of the embryo to implant onto the side of the uterus wall following IVF treatment. Regularly, this happens at 6–7 days after conception and involves the embedding of the growing embryo into the mothers uterus and a connection being formed. A successful implantation can be determined by using an ultrasound to view the sac which the baby grows in, inside the uterus.

Preimplantation factor(PIF) sometimes styled PreImplantation Factor is a peptide secreted by trophoblast cells prior to placenta formation in early embryonic development. Human embryos begin to express PIF at the 4-cell stage, with expression increasing by the morula stage and continuing to do so throughout the first trimester. Expression of preimplantation factor in the blastocyst was discovered as an early correlate of the viability of the eventual pregnancy. Preimplantation factor was identified in 1994 by a lymphocyte platelet-binding assay, where it was thought to be an early biomarker of pregnancy. It has a simple primary structure with a short sequence of fifteen amino acids without any known quaternary structure. A synthetic analogue of preimplantation factor that has an identical amino acid sequence and mimics the normal biological activity of PIF has been developed and is commonly used in research studies, particularly those that aim to study potential adult therapeutics.

Maternal recognition of pregnancy is a crucial aspect of carrying a pregnancy to full term. Without maternal recognition to maintain pregnancy, the initial messengers which stop luteolysis and promote foetal implantation, growth and uterine development finish with nothing to replace them and the pregnancy is lost.











