Androgen synthesis
Theca cells are responsible for synthesizing androgens, providing signal transduction between granulosa cells and oocytes during development by the establishment of a vascular system, providing nutrients, and providing structure and support to the follicle as it matures. [2]

Theca cells are responsible for the production of androstenedione, and indirectly the production of 17β estradiol, also called E2, by supplying the neighboring granulosa cells with androstenedione that with the help of the enzyme aromatase can be used as a substrate for this type of estradiol. [3] FSH induces the granulosa cells to synthesize aromatase, an enzyme that converts the androgens made by the theca interna into estradiol. [3]
Signaling cascade
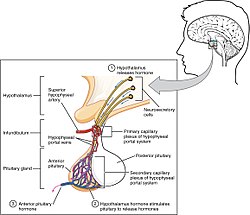
Gonadotropin releasing hormone (GnRH) is released by projections of the hypothalamus into the anterior pituitary gland. Gonadotrophs are stimulated to produce follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which are released into the bloodstream to act upon the ovaries. Luteinizing hormone serves to directly stimulate theca cells. Together, these organs comprise the HPG axis.
Within the ovaries, transmembrane G-protein coupled receptors (GPCRs) bind to LH in the bloodstream, and the signal is transduced to the interior of theca cells through the action of the second messenger cAMP and third messenger protein kinase A (PKA). Theca cells are then stimulated to produce testosterone, which is sent in a paracrine fashion to neighboring granulosa cells for conversion to estradiol. [4]
Disorders
Hyperactivity of theca cells causes hyperandrogenism, and hypoactivity leads to a lack of estrogen. [5] Granulosa cell tumors, while rare (less than 5% of ovarian cancers), may both granulosa cells and theca cells. [6] Thecomas are benign proliferations of theca cells that may present with hormonal dysfunction. [7]
Theca cells (along with granulosa cells) form the corpus luteum during oocyte maturation. Theca cells are only correlated with developing ovarian follicles. [5] They are the leading cause of endocrine-based infertility, as either hyperactivity or hypoactivity of the theca cells can lead to fertility problems.
