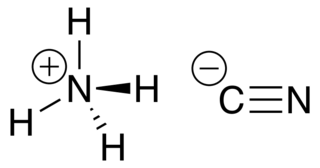In chemistry, cyanide is a chemical compound that contains a C≡N functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.

The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using a finely divided iron metal catalyst:

The Miller–Urey experiment, or Miller experiment, was an experiment in chemical synthesis carried out in 1952 that simulated the conditions thought at the time to be present in the atmosphere of the early, prebiotic Earth. It is seen as one of the first successful experiments demonstrating the synthesis of organic compounds from inorganic constituents in an origin of life scenario. The experiment used methane (CH4), ammonia (NH3), hydrogen (H2), in ratio 2:2:1, and water (H2O). Applying an electric arc (simulating lightning) resulted in the production of amino acids.
The Ostwald process is a chemical process used for making nitric acid (HNO3). The Ostwald process is a mainstay of the modern chemical industry, and it provides the main raw material for the most common type of fertilizer production. Historically and practically, the Ostwald process is closely associated with the Haber process, which provides the requisite raw material, ammonia (NH3). This method is preferred over other methods of nitric acid production, in that it is less expensive and more efficient.
Hydrogen cyanide is a chemical compound with the formula HCN and structural formula H−C≡N. It is a highly toxic and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. A solution of hydrogen cyanide in water, represented as HCN, is called hydrocyanic acid. The salts of the cyanide anion are known as cyanides.

Sodium cyanide is a compound with the formula NaCN and the structure Na+−C≡N. It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. It is a moderately strong base.

Hydrazine is an inorganic compound with the chemical formula N2H4. It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydrazine hydrate.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
Benzonitrile is the chemical compound with the formula C6H5(CN), abbreviated PhCN. This aromatic organic compound is a colorless liquid with a sweet bitter almond odour. It is mainly used as a precursor to the resin benzoguanamine.

Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, and in the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers. Some astrobiologists suggest that it may be an alternative to water as the main solvent in other forms of life.

The Sabatier reaction or Sabatier process produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures and pressures in the presence of a nickel catalyst. It was discovered by the French chemists Paul Sabatier and Jean-Baptiste Senderens in 1897. Optionally, ruthenium on alumina makes a more efficient catalyst. It is described by the following exothermic reaction:
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with cyanide in the presence of ammonia. The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid. The method is used for the commercial production of racemic methionine from methional.

Leonid Andrussow was a German chemical engineer. He developed the process for the production of hydrogen cyanide based on the oxidation of ammonia and methane, which is named after him Andrussow oxidation.
The BMA process or Degussa process is a chemical process developed by the German chemical company Degussa for the production of hydrogen cyanide from methane and ammonia in presence of a platinum catalyst. Hydrogen cyanide is used in the chemical industry for the production of intermediate chemicals like acrylonitrile, methyl methacrylate, and adiponitrile.
Methanation is the conversion of carbon monoxide and carbon dioxide (COx) to methane (CH4) through hydrogenation. The methanation reactions of COx were first discovered by Sabatier and Senderens in 1902.
PROX is an acronym for PReferential OXidation, that refers to the preferential oxidation of carbon monoxide in a gas mixture by a catalyst. It is intended to remove trace amounts of CO from H2/CO/CO2 mixtures produced by steam reforming and water-gas shift. An ideal PROX catalyst preferentially oxidizes carbon monoxide (CO) using a heterogeneous catalyst placed upon a ceramic support. Catalysts include metals such as platinum, platinum/iron, platinum/ruthenium, gold nanoparticles as well as novel copper oxide/ceramic conglomerate catalysts.
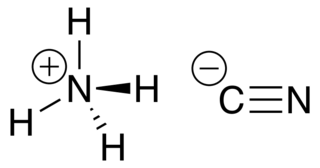
Ammonium cyanide is an unstable inorganic compound with the formula NH4CN.
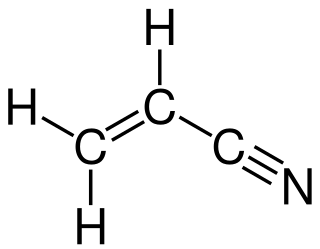
In organic chemistry, ammoxidation is a process for the production of nitriles using ammonia and oxygen. It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio. The usual substrates are alkenes. Several million tons of acrylonitrile are produced in this way annually:
Methanizer is an appliance used in gas chromatography (GC), which allows the user to detect very low concentrations of carbon monoxide and carbon dioxide. It consists of a flame ionization detector, preceded by a hydrogenating reactor, which converts CO2 and CO into methane CH4. Methanizers contain a hydrogenation catalyst to achieve this conversion. Nickel is commonly used as the catalyst and there are alternatives available.

Acetone imine, or 2-propanimine is an organic compound and an imine with the chemical formula (CH3)2CNH. It is a volatile and flammable liquid at room temperature. It is the simplest ketimine. This compound is mainly of academic interest.