
The aldol reaction is a reaction in organic chemistry that combines two carbonyl compounds to form a new β-hydroxy carbonyl compound. Its simplest form might involve the nucleophilic addition of an enolized ketone to another:

An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties react to form a β-hydroxyaldehyde or β-hydroxyketone, and this is then followed by dehydration to give a conjugated enone.
The Perkin reaction is an organic reaction developed by English chemist William Henry Perkin that is used to make cinnamic acids. It gives an α,β-unsaturated aromatic acid or α-substituted β-aryl acrylic acid by the aldol condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid. The alkali salt acts as a base catalyst, and other bases can be used instead.
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.
In organic chemistry, the Knoevenagel condensation reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation.
The Pictet–Spengler reaction is a chemical reaction in which a β-arylethylamine undergoes condensation with an aldehyde or ketone followed by ring closure. The reaction was first discovered in 1911 by Amé Pictet and Theodor Spengler. Traditionally, an acidic catalyst in protic solvent was employed with heating; however, the reaction has been shown to work in aprotic media in superior yields and sometimes without acid catalysis. The Pictet–Spengler reaction can be considered a special case of the Mannich reaction, which follows a similar reaction pathway. The driving force for this reaction is the electrophilicity of the iminium ion generated from the condensation of the aldehyde and amine under acid conditions. This explains the need for an acid catalyst in most cases, as the imine is not electrophilic enough for ring closure but the iminium ion is capable of undergoing the reaction.

The Bamford–Stevens reaction is a chemical reaction whereby treatment of tosylhydrazones with strong base gives alkenes. It is named for the British chemist William Randall Bamford and the Scottish chemist Thomas Stevens Stevens (1900–2000). The usage of aprotic solvents gives predominantly Z-alkenes, while protic solvent gives a mixture of E- and Z-alkenes. As an alkene-generating transformation, the Bamford–Stevens reaction has broad utility in synthetic methodology and complex molecule synthesis.
The Japp–Klingemann reaction is a chemical reaction used to synthesize hydrazones from β-keto-acids and aryl diazonium salts. The reaction is named after the chemists Francis Robert Japp and Felix Klingemann.
The Ullmann reaction or Ullmann coupling, named after Fritz Ullmann, couples two aryl or alkyl groups with the help of copper. The reaction was first reported by Ullmann and his student Bielecki in 1901. It has been later shown that palladium and nickel can also be effectively used.

The Petasis reaction is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.

Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.
The McFadyen–Stevens reaction is a chemical reaction best described as a base-catalyzed thermal decomposition of acylsulfonylhydrazides to aldehydes.

In organic chemistry, the Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde or formate. The reaction was discovered by Teruaki Mukaiyama in 1973. His choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone, or a different aldehyde without self-condensation of the aldehyde. For this reason the reaction is used extensively in organic synthesis.
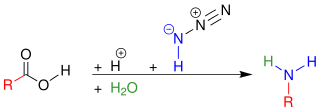
In organic chemistry, the Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually an aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine or amide, with expulsion of nitrogen. It is named after Karl Friedrich Schmidt (1887–1971), who first reported it in 1924 by successfully converting benzophenone and hydrazoic acid to benzanilide. The intramolecular reaction was not reported until 1991 but has become important in the synthesis of natural products. The reaction is effective with carboxylic acids to give amines (above), and with ketones to give amides (below).
In organic chemistry, the Baylis–Hillman, Morita–Baylis–Hillman, or MBH reaction is a carbon-carbon bond-forming reaction between an activated alkene and a carbon electrophile in the presence of a nucleophilic catalyst, such as a tertiary amine or phosphine. The product is densely functionalized, joining the alkene at the α-position to a reduced form of the electrophile.
The Mozingo reduction, also known as Mozingo reaction or thioketal reduction, is a chemical reaction capable of fully reducing a ketone or aldehyde to the corresponding alkane via a dithioacetal. The reaction scheme is as follows:

Hydroxylamine-O-sulfonic acid (HOSA) or aminosulfuric acid is the inorganic compound with molecular formula H3NO4S that is formed by the sulfonation of hydroxylamine with oleum. It is a white, water-soluble and hygroscopic, solid, commonly represented by the condensed structural formula H2NOSO3H, though it actually exists as a zwitterion and thus is more accurately represented as +H3NOSO3−. It is used as a reagent for the introduction of amine groups (–NH2), for the conversion of aldehydes into nitriles and alicyclic ketones into lactams (cyclic amides), and for the synthesis of variety of nitrogen-containing heterocycles.
In organophosphorus chemistry, the Pudovik reaction is a method for preparing α-aminomethylphosphonates. Under basic conditions, the phosphorus–hydrogen bond of a dialkylphosphite, (RO)2P(O)H, adds across the carbon–nitrogen double bond of an imine (a hydrophosphonylation reaction). The reaction is closely related to the three-component Kabachnik–Fields reaction, where an amine, phosphite, and an organic carbonyl compound are condensed, which was reported independently by Martin Kabachnik and Ellis Fields in 1952. In the Pudovik reaction, a generic imine, RCH=NR', would react with a phosphorous reagent like diethylphosphite as follows:

2-Carboxybenzaldehyde is a chemical compound. It consists of a benzene ring, with an aldehyde and a carboxylic acid as substituents that are ortho to each other. The compound exhibits ring–chain tautomerism: the two substituents can react with each other to form 3-hydroxyphthalide, a cyclic lactol. This lactol reacts readily with Grignard reagents, forming alkyl- and aryl-substituted phthalides. Other benzo-fused heterocyclic compounds can be derived from 2-carboxybenzaldehyde, including isoindolinones and phthalazinones, with a variety of pharmacological properties, such as the antihistamine azelastine.












