
A brain tumor occurs when abnormal cells form within the brain. There are two main types of tumors: malignant (cancerous) tumors and benign (non-cancerous) tumors. These can be further classified as primary tumors, which start within the brain, and secondary tumors, which most commonly have spread from tumors located outside the brain, known as brain metastasis tumors. All types of brain tumors may produce symptoms that vary depending on the size of the tumor and the part of the brain that is involved. Where symptoms exist, they may include headaches, seizures, problems with vision, vomiting and mental changes. Other symptoms may include difficulty walking, speaking, with sensations, or unconsciousness.

A glioma is a type of primary tumor that starts in the glial cells of the brain or spinal cord. They are cancerous but some are extremely slow to develop. Gliomas comprise about 30 percent of all brain tumors and central nervous system tumours, and 80 percent of all malignant brain tumours.

Oligodendrogliomas are a type of glioma that are believed to originate from the oligodendrocytes of the brain or from a glial precursor cell. They occur primarily in adults but are also found in children.

Glioblastoma, previously known as glioblastoma multiforme (GBM), is the most aggressive and most common type of cancer that originates in the brain, and has a very poor prognosis for survival. Initial signs and symptoms of glioblastoma are nonspecific. They may include headaches, personality changes, nausea, and symptoms similar to those of a stroke. Symptoms often worsen rapidly and may progress to unconsciousness.

Isocitrate dehydrogenase (IDH) (EC 1.1.1.42) and (EC 1.1.1.41) is an enzyme that catalyzes the oxidative decarboxylation of isocitrate, producing alpha-ketoglutarate (α-ketoglutarate) and CO2. This is a two-step process, which involves oxidation of isocitrate (a secondary alcohol) to oxalosuccinate (a ketone), followed by the decarboxylation of the carboxyl group beta to the ketone, forming alpha-ketoglutarate. In humans, IDH exists in three isoforms: IDH3 catalyzes the third step of the citric acid cycle while converting NAD+ to NADH in the mitochondria. The isoforms IDH1 and IDH2 catalyze the same reaction outside the context of the citric acid cycle and use NADP+ as a cofactor instead of NAD+. They localize to the cytosol as well as the mitochondrion and peroxisome.

Succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial (SDHB) also known as iron-sulfur subunit of complex II (Ip) is a protein that in humans is encoded by the SDHB gene.
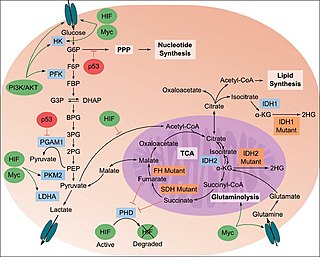
The study of the tumor metabolism, also known as tumor metabolome describes the different characteristic metabolic changes in tumor cells. The characteristic attributes of the tumor metabolome are high glycolytic enzyme activities, the expression of the pyruvate kinase isoenzyme type M2, increased channeling of glucose carbons into synthetic processes, such as nucleic acid, amino acid and phospholipid synthesis, a high rate of pyrimidine and purine de novo synthesis, a low ratio of Adenosine triphosphate and Guanosine triphosphate to Cytidine triphosphate and Uridine triphosphate, low Adenosine monophosphate levels, high glutaminolytic capacities, release of immunosuppressive substances and dependency on methionine.

Ollier disease is a rare sporadic nonhereditary skeletal disorder in which typically benign cartilaginous tumors (enchondromas) develop near the growth plate cartilage. This is caused by cartilage rests that grow and reside within the metaphysis or diaphysis and eventually mineralize over time to form multiple enchondromas. Key signs of the disorder include asymmetry and shortening of the limb as well as an increased thickness of the bone margin. These symptoms are typically first visible during early childhood with the mean age of diagnosis being 13 years of age. Many patients with Ollier disease are prone to develop other malignancies including bone sarcomas that necessitate treatment and the removal of malignant bone neoplasm. Cases in patients with Ollier disease has shown a link to IDH1, IDH2, and PTH1R gene mutations. Currently, there are no forms of treatment for the underlying condition of Ollier disease but complications such as fractures, deformities, malignancies that arise from it can be treated through surgical procedures. The prevalence of this condition is estimated at around 1 in 100,000. It is unclear whether the men or women are more affected by this disorder due to conflicting case studies.

Oligodendrocyte transcription factor (OLIG2) is a basic helix-loop-helix (bHLH) transcription factor encoded by the OLIG2 gene. The protein is of 329 amino acids in length, 32 kDa in size and contains one basic helix-loop-helix DNA-binding domain. It is one of the three members of the bHLH family. The other two members are OLIG1 and OLIG3. The expression of OLIG2 is mostly restricted in central nervous system, where it acts as both an anti-neurigenic and a neurigenic factor at different stages of development. OLIG2 is well known for determining motor neuron and oligodendrocyte differentiation, as well as its role in sustaining replication in early development. It is mainly involved in diseases such as brain tumor and Down syndrome.

Isocitrate dehydrogenase [NAD] subunit alpha, mitochondrial (IDH3α) is an enzyme that in humans is encoded by the IDH3A gene.

Isocitrate dehydrogenase [NADP], mitochondrial is an enzyme that in humans is encoded by the IDH2 gene.

Isocitrate dehydrogenase [NAD] subunit gamma, mitochondrial is an enzyme that in humans is encoded by the IDH3G gene.

Isocitrate dehydrogenase [NAD] subunit beta, mitochondrial is an enzyme that in humans is encoded by the IDH3B gene.

α-Hydroxyglutaric acid is an alpha hydroxy acid form of glutaric acid.

Temozolomide, sold under the brand name Temodar among others, is an anticancer medication used to treat brain tumors such as glioblastoma and anaplastic astrocytoma. It is taken by mouth or via intravenous infusion.

Tet methylcytosine dioxygenase 2 (TET2) is a human gene. It resides at chromosome 4q24, in a region showing recurrent microdeletions and copy-neutral loss of heterozygosity (CN-LOH) in patients with diverse myeloid malignancies.

Branched chain amino acid transaminase 1 is a protein that in humans is encoded by the BCAT1 gene. It is the first enzyme in the branched-chain amino acid (BCAA) degradation pathway and facilitates the reversible transamination of BCAAs and glutamate. BCAT1 resides in the cytoplasm, while its isoform, BCAT2, is found in the mitochondria.

Ivosidenib, sold under the brand name Tibsovo, is an anti-cancer medication for the treatment of acute myeloid leukemia (AML) and cholangiocarcinoma. It is a small molecule inhibitor of isocitrate dehydrogenase-1 (IDH1), which is mutated in several forms of cancer. Ivosidenib is an isocitrate dehydrogenase-1 inhibitor that works by decreasing abnormal production of the oncometabolite 2-hydroxyglutarate (2-HG), leading to differentiation of malignant cells.

Oncometabolism is the field of study that focuses on the metabolic changes that occur in cells that make up the tumor microenvironment (TME) and accompany oncogenesis and tumor progression toward a neoplastic state.
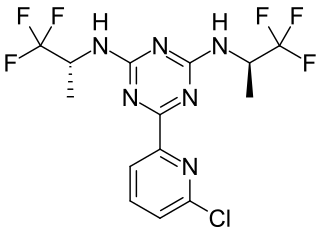
Vorasidenib, sold under the brand name Voranigo, is an anti-cancer medication used for the treatment of certain forms of glioma. Vorasidenib is a dual mutant isocitrate dehydrogenase 1 and isocitrate dehydrogenase 2 (mIDH1/2) inhibitor.























