
Interferons are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.

The hepatitis C virus (HCV) is a small, enveloped, positive-sense single-stranded RNA virus of the family Flaviviridae. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer and lymphomas in humans.
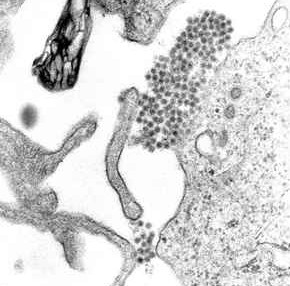
Dengue virus (DENV) is the cause of dengue fever. It is a mosquito-borne, single positive-stranded RNA virus of the family Flaviviridae; genus Flavivirus. Four serotypes of the virus have been found, and a reported fifth has yet to be confirmed, all of which can cause the full spectrum of disease. Nevertheless, scientists' understanding of dengue virus may be simplistic as, rather than distinct antigenic groups, a continuum appears to exist. This same study identified 47 strains of dengue virus. Additionally, coinfection with and lack of rapid tests for Zika virus and chikungunya complicate matters in real-world infections.
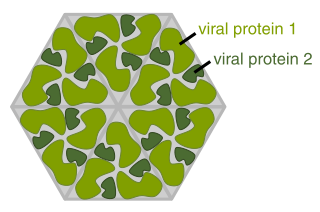
A viral protein is both a component and a product of a virus. Viral proteins are grouped according to their functions, and groups of viral proteins include structural proteins, nonstructural proteins, regulatory proteins, and accessory proteins. Viruses are non-living and do not have the means to reproduce on their own, instead depending on their host cell's resources in order to reproduce. Thus, viruses do not code for many of their own viral proteins, and instead use the host cell's machinery to produce the viral proteins they require for replication.
An oncovirus or oncogenic virus is a virus that can cause cancer. This term originated from studies of acutely transforming retroviruses in the 1950–60s, when the term "oncornaviruses" was used to denote their RNA virus origin. With the letters "RNA" removed, it now refers to any virus with a DNA or RNA genome causing cancer and is synonymous with "tumor virus" or "cancer virus". The vast majority of human and animal viruses do not cause cancer, probably because of longstanding co-evolution between the virus and its host. Oncoviruses have been important not only in epidemiology, but also in investigations of cell cycle control mechanisms such as the retinoblastoma protein.

The Matrix-2 (M2) protein is a proton-selective viroporin, integral in the viral envelope of the influenza A virus. The channel itself is a homotetramer, where the units are helices stabilized by two disulfide bonds, and is activated by low pH. The M2 protein is encoded on the seventh RNA segment together with the M1 protein. Proton conductance by the M2 protein in influenza A is essential for viral replication.
The NS1 influenza protein (NS1) is a viral nonstructural protein encoded by the NS gene segments of type A, B and C influenza viruses. Also encoded by this segment is the nuclear export protein (NEP), formally referred to as NS2 protein, which mediates the export of influenza virus ribonucleoprotein (RNP) complexes from the nucleus, where they are assembled.

RNA-dependent RNA polymerase (RdRp) or RNA replicase is an enzyme that catalyzes the replication of RNA from an RNA template. Specifically, it catalyzes synthesis of the RNA strand complementary to a given RNA template. This is in contrast to typical DNA-dependent RNA polymerases, which all organisms use to catalyze the transcription of RNA from a DNA template.
Yizhi Jane Tao is a Chinese biochemist, structural biologist, and professor of biochemistry and cell biology at Rice University in Houston, Texas. Professor Tao led a team of researchers to be the first to map the structure of the influenza A virus nucleoprotein to an atomic level, a feat which circulated widely in the popular press. She was named among the top ten most influential Chinese of 2006 by a consortium of China's leading media outlets including Phoenix Satellite Television, China News Service, Asia Newsweek, and World Journal.

Mitochondrial antiviral-signaling protein (MAVS) is a protein that is essential for antiviral innate immunity. MAVS is located in the outer membrane of the mitochondria, peroxisomes, and mitochondrial-associated endoplasmic reticulum membrane (MAM). Upon viral infection, a group of cytosolic proteins will detect the presence of the virus and bind to MAVS, thereby activating MAVS. The activation of MAVS leads the virally infected cell to secrete cytokines. This induces an immune response which kills the host's virally infected cells, resulting in clearance of the virus.

VAMP-Associated Protein A is a protein that in humans is encoded by the VAPA gene. Together with VAPB and VAPC it forms the VAP protein family. They are integral endoplasmic reticulum membrane proteins of the type II and are ubiquitous among eukaryotes.
NS2-3 protease is an enzyme responsible for proteolytic cleavage between NS2 and NS3, which are non-structural proteins that form part of the HCV virus particle. NS3 protease of hepatitis C virus, on the other hand, is responsible for the cleavage of non-structural protein downstream. Both of these proteases are directly involved in HCV genome replication, that is, during the viral life-cycle that leads to virus multiplication in the host that has been infected by the virus.

Nonstructural protein 3 (NS3), also known as p-70, is a viral nonstructural protein that is 70 kDa cleavage product of the hepatitis C virus polyprotein. It acts as a serine protease. C-terminal two-thirds of the protein also acts as helicase and nucleoside triphosphatase. First (N-terminal) 180 aminoacids of NS3 has additional role as cofactor domains for NS2 protein.

Nonstructural protein 5A (NS5A) is a zinc-binding and proline-rich hydrophilic phosphoprotein that plays a key role in Hepatitis C virus RNA replication. It appears to be a dimeric form without trans-membrane helices.
A hepatitis C vaccine, a vaccine capable of protecting against the hepatitis C virus (HCV), is not yet available. Although vaccines exist for hepatitis A and hepatitis B, development of an HCV vaccine has presented challenges. No vaccine is currently available, but several vaccines are currently under development.
RIG-I-like receptors are a type of intracellular pattern recognition receptor involved in the recognition of viruses by the innate immune system. RIG-I is the best characterized receptor within the RIG-I like receptor (RLR) family. Together with MDA5 and LGP2, this family of cytoplasmic pattern recognition receptors (PRRs) are sentinels for intracellular viral RNA that is a product of viral infection. The RLR receptors provide frontline defence against viral infections in most tissues.

Radical S-adenosyl methionine domain-containing protein 2 is a protein that in humans is encoded by the RSAD2 gene. RSAD2 is a multifunctional protein in viral processes that is an interferon stimulated gene. It has been reported that viperin could be induced by either IFN-dependent or IFN-independent pathways and certain viruses may use viperin to increase their infectivity.

Daclatasvir, sold under the brand name Daklinza, is an antiviral medication used in combination with other medications to treat hepatitis C (HCV). The other medications used in combination include sofosbuvir, ribavirin, and interferon, vary depending on the virus type and whether the person has cirrhosis. It is taken by mouth.

Nonstructural protein 5B (NS5B) is a viral protein found in the hepatitis C virus (HCV). It is an RNA-dependent RNA polymerase, having the key function of replicating HCV's viral RNA by using the viral positive RNA strand as a template to catalyze the polymerization of ribonucleoside triphosphates (rNTP) during RNA replication. Several crystal structures of NS5B polymerase in several crystalline forms have been determined based on the same consensus sequence BK. The structure can be represented by a right hand shape with fingers, palm, and thumb. The encircled active site, unique to NS5B, is contained within the palm structure of the protein. Recent studies on NS5B protein genotype 1b strain J4's (HC-J4) structure indicate a presence of an active site where possible control of nucleotide binding occurs and initiation of de-novo RNA synthesis. De-novo adds necessary primers for initiation of RNA replication.

Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs.












