
Hepadnaviridae is a family of viruses. Humans, apes, and birds serve as natural hosts. There are currently 18 species in this family, divided among 5 genera. Its best-known member is hepatitis B virus. Diseases associated with this family include: liver infections, such as hepatitis, hepatocellular carcinomas, and cirrhosis. It is the sole accepted family in the order Blubervirales.
Papillomaviridae is a family of non-enveloped DNA viruses whose members are known as papillomaviruses. Several hundred species of papillomaviruses, traditionally referred to as "types", have been identified infecting all carefully inspected mammals, but also other vertebrates such as birds, snakes, turtles and fish. Infection by most papillomavirus types, depending on the type, is either asymptomatic or causes small benign tumors, known as papillomas or warts. Papillomas caused by some types, however, such as human papillomaviruses 16 and 18, carry a risk of becoming cancerous.
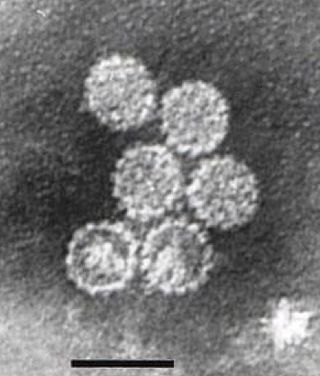
SV40 is an abbreviation for simian vacuolating virus 40 or simian virus 40, a polyomavirus that is found in both monkeys and humans. Like other polyomaviruses, SV40 is a DNA virus that sometimes causes tumors in animals, but most often persists as a latent infection. SV40 has been widely studied as a model eukaryotic virus, leading to many early discoveries in eukaryotic DNA replication and transcription.
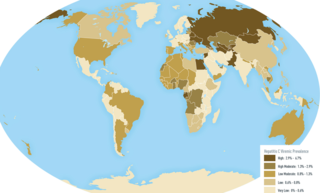
An oncovirus or oncogenic virus is a virus that can cause cancer. This term originated from studies of acutely transforming retroviruses in the 1950–60s, when the term "oncornaviruses" was used to denote their RNA virus origin. With the letters "RNA" removed, it now refers to any virus with a DNA or RNA genome causing cancer and is synonymous with "tumor virus" or "cancer virus". The vast majority of human and animal viruses do not cause cancer, probably because of longstanding co-evolution between the virus and its host. Oncoviruses have been important not only in epidemiology, but also in investigations of cell cycle control mechanisms such as the retinoblastoma protein.
A nuclear localization signalorsequence (NLS) is an amino acid sequence that 'tags' a protein for import into the cell nucleus by nuclear transport. Typically, this signal consists of one or more short sequences of positively charged lysines or arginines exposed on the protein surface. Different nuclear localized proteins may share the same NLS. An NLS has the opposite function of a nuclear export signal (NES), which targets proteins out of the nucleus.
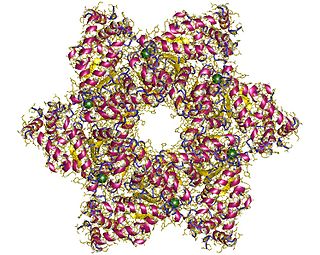
SV40 large T antigen is a hexamer protein that is a dominant-acting oncoprotein derived from the polyomavirus SV40. TAg is capable of inducing malignant transformation of a variety of cell types. The transforming activity of TAg is due in large part to its perturbation of the retinoblastoma (pRb) and p53 tumor suppressor proteins. In addition, TAg binds to several other cellular factors, including the transcriptional co-activators p300 and CBP, which may contribute to its transformation function. Similar proteins from related viruses are known as large tumor antigen in general.
Paul Ahlquist is an American virologist who is Professor of Oncology, Molecular Virology, and Plant Pathology at the University of Wisconsin–Madison. He is the Associate Director of Basic Sciences at the University of Wisconsin Carbone Cancer Center and the Director of the John and Jeanne Rowe Center for Research in Virology at the Morgridge Institute for Research.

Herpes simplex virus1 and 2, also known by their taxonomic names Human alphaherpesvirus 1 and Human alphaherpesvirus 2, are two members of the human Herpesviridae family, a set of viruses that produce viral infections in the majority of humans. Both HSV-1 and HSV-2 are very common and contagious. They can be spread when an infected person begins shedding the virus.

Bovine papillomaviruses (BPV) are a paraphyletic group of DNA viruses of the subfamily Firstpapillomavirinae of Papillomaviridae that are common in cattle. All BPVs have a circular double-stranded DNA genome. Infection causes warts of the skin and alimentary tract, and more rarely cancers of the alimentary tract and urinary bladder. They are also thought to cause the skin tumour equine sarcoid in horses and donkeys.

The Shope papilloma virus (SPV), also known as cottontail rabbit papilloma virus (CRPV) or Kappapapillomavirus 2, is a papillomavirus which infects certain leporids, causing keratinous carcinomas resembling horns, typically on or near the animal's head. The carcinomas can metastasize or become large enough to interfere with the host's ability to eat, causing starvation. Richard E. Shope investigated the horns and discovered the virus in 1933, an important breakthrough in the study of oncoviruses. The virus was originally discovered in cottontail rabbits in the Midwestern U.S. but can also infect brush rabbits, black-tailed jackrabbits, snowshoe hares, European rabbits, and domestic rabbits.

Importin subunit alpha-1 is a protein that in humans is encoded by the KPNA2 gene.

Importin subunit beta-1 is a protein that in humans is encoded by the KPNB1 gene.

Importin subunit alpha-5 is a protein that in humans is encoded by the KPNA1 gene.

Importin subunit alpha-7 is a protein that in humans is encoded by the KPNA6 gene.

Importin subunit alpha-3, also known as karyopherin subunit alpha-4, is a protein that in humans is encoded by the KPNA4 gene.

Importin subunit alpha-6 is a protein that in humans is encoded by the KPNA5 gene.

Transportin-1 is a protein that in humans is encoded by the TNPO1 gene.
Importin alpha, or karyopherin alpha refers to a class of adaptor proteins that are involved in the import of proteins into the cell nucleus. They are a sub-family of karyopherin proteins.

Major capsid protein VP1 is a viral protein that is the main component of the polyomavirus capsid. VP1 monomers are generally around 350 amino acids long and are capable of self-assembly into an icosahedral structure consisting of 360 VP1 molecules organized into 72 pentamers. VP1 molecules possess a surface binding site that interacts with sialic acids attached to glycans, including some gangliosides, on the surfaces of cells to initiate the process of viral infection. The VP1 protein, along with capsid components VP2 and VP3, is expressed from the "late region" of the circular viral genome.
Minor capsid protein VP2 and minor capsid protein VP3 are viral proteins that are components of the polyomavirus capsid. Polyomavirus capsids are composed of three proteins; the major component is major capsid protein VP1, which self-assembles into pentamers that in turn self-assemble into enclosed icosahedral structures. The minor components are VP2 and VP3, which bind in the interior of the capsid.















