Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses.

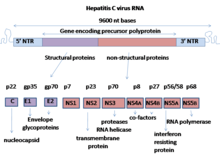
The hepatitis C virus (HCV) is a small, enveloped, positive-sense single-stranded RNA virus of the family Flaviviridae. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer and lymphomas in humans.

RNA-dependent RNA polymerase (RdRp) or RNA replicase is an enzyme that catalyzes the replication of RNA from an RNA template. Specifically, it catalyzes synthesis of the RNA strand complementary to a given RNA template. This is in contrast to typical DNA-dependent RNA polymerases, which all organisms use to catalyze the transcription of RNA from a DNA template.

This family represents the internal ribosome entry site (IRES) of the hepatitis A virus. HAV IRES is a 450 nucleotide long sequence located in the 735 nt long 5’ UTR of Hepatitis A viral RNA genome. IRES elements allow cap and end-independent translation of mRNA in the host cell. The IRES achieves this by mediating the internal initiation of translation by recruiting a ribosomal 40S pre-initiation complex directly to the initiation codon and eliminates the requirement for eukaryotic initiation factor, eIF4F.

Nonstructural protein 5A (NS5A) is a zinc-binding and proline-rich hydrophilic phosphoprotein that plays a key role in Hepatitis C virus RNA replication. It appears to be a dimeric form without trans-membrane helices.

Nonstructural protein 2 (NS2) is a viral protein found in the hepatitis C virus. It is also produced by influenza viruses, and is alternatively known as the nuclear export protein (NEP).

Hepatitis B virus DNA polymerase is a hepatitis B viral protein. It is a DNA polymerase that can use either DNA or RNA templates and a ribonuclease H that cuts RNA in the duplex. Both functions are supplied by the reverse transcriptase (RT) domain.

Hepatitis B virus (HBV) is a partially double-stranded DNA virus, a species of the genus Orthohepadnavirus and a member of the Hepadnaviridae family of viruses. This virus causes the disease hepatitis B.
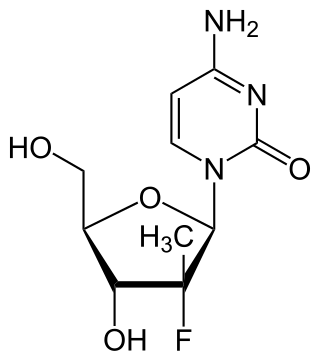
PSI-6130 is an experimental treatment for hepatitis C. PSI-6130 is a member of a class of antiviral drugs known as nucleoside polymerase inhibitors that was created by chemist Jeremy L. Clark. Specifically, PSI-6130 inhibits the hepatitis C virus RNA dependant RNA polymerase called NS5B.
Ledipasvir is a drug for the treatment of hepatitis C that was developed by Gilead Sciences. After completing Phase III clinical trials, on February 10, 2014, Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin.
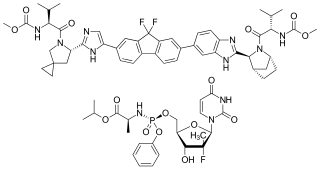
Ledipasvir/sofosbuvir, sold under the trade name Harvoni among others, is a medication used to treat hepatitis C. It is a fixed-dose combination of ledipasvir and sofosbuvir. Cure rates are 94% to 99% in people infected with hepatitis C virus (HCV) genotype 1. Some evidence also supports use in HCV genotype 3 and 4. It is taken daily by mouth for 8–24 weeks.
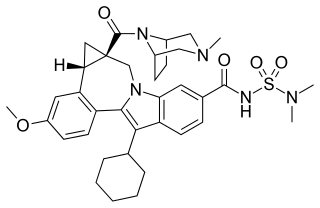
Beclabuvir is an antiviral drug for the treatment of hepatitis C virus (HCV) infection that has been studied in clinical trials. In February 2017, Bristol-Myers Squibb began sponsoring a post-marketing trial of beclabuvir, in combination with asunaprevir and daclatasvir, to study the combination's safety profile with regard to liver function. From February 2014 to November 2016, a phase II clinical trial was conducted on the combination of asunaprevir/daclatasvir/beclabuvir on patients infected with both HIV and HCV. Furthermore, a recent meta-analysis of six published six clinical trials showed high response rates in HCV genotype 1-infected patients treated with daclatasvir, asunaprevir, and beclabuvir irrespective of ribavirin use, prior interferon-based therapy, or restriction on noncirrhotic patients, IL28B genotype, or baseline resistance-associated variants
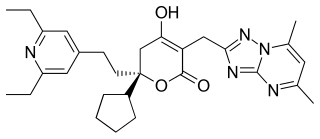
Filibuvir was a non-nucleoside orally available NS5B inhibitor developed by Pfizer for the treatment of hepatitis C. It binds to the non-catalytic Thumb II allosteric pocket of NS5B viral polymerase and causes a decrease in viral RNA synthesis. It is a potent and selective inhibitor, with a mean IC50 of 0.019 μM against genotype 1 polymerases. Several filibuvir-resistant mutations have been identified, M423 being the most common that occurred after filibuvir monotherapy. It was intended to be taken twice-daily.

Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs.

Mericitabine (RG-7128) is an antiviral drug, a deoxycytidine analog. It was developed as a treatment for hepatitis C, acting as a NS5B RNA polymerase inhibitor, but while it showed a good safety profile in clinical trials, it was not sufficiently effective to be used as a stand-alone agent. However mericitabine has been shown to boost the efficacy of other antiviral drugs when used alongside them, and as most modern treatment regimens for hepatitis C use a combination therapy of several antiviral drugs, clinical trials have continued to see if it can form a part of a clinically useful drug treatment program.
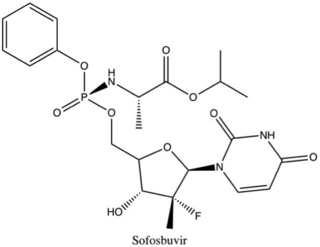
Non-structural protein 5B (NS5B) inhibitors are a class of direct-acting antivirals widely used in the treatment of chronic hepatitis C. Depending on site of action and chemical composition, NS5B inhibitors may be categorized into three classes—nucleoside active site inhibitors (NIs), non-nucleoside allosteric inhibitors, and pyrophosphate analogues. Subsequently, all three classes are then subclassified. All inhibit RNA synthesis by NS5B but at different stages/sites resulting in inability of viral RNA replication. Expression of direct-acting NS5B inhibitors does not take place in cells that are not infected by hepatitis C virus, which seems to be beneficial for this class of drugs.
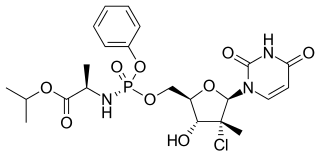
Uprifosbuvir (MK-3682) is an antiviral drug developed for the treatment of hepatitis C. It is a nucleotide analogue which acts as an NS5B RNA polymerase inhibitor. As of 2019 it was in Phase III human clinical trials.
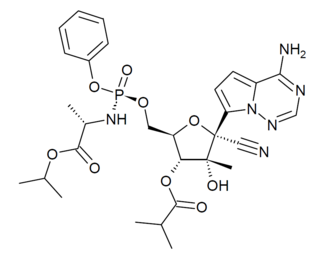
GS-6620 is an antiviral drug which is a nucleotide analogue. It was developed for the treatment of Hepatitis C but while it showed potent antiviral effects in early testing, it could not be successfully formulated into an oral dosage form due to low and variable absorption in the intestines which made blood levels unpredictable. It has however continued to be researched as a potential treatment for other viral diseases such as Ebola virus disease.
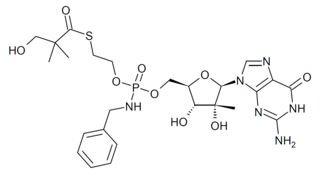
IDX-184 is an antiviral drug which was developed as a treatment for hepatitis C, acting as a NS5B RNA polymerase inhibitor. While it showed reasonable effectiveness in early clinical trials it did not progress past Phase IIb. However research using this drug has continued as it shows potentially useful activity against other emerging viral diseases such as Zika virus, and coronaviruses including MERS, and SARS-CoV-2.
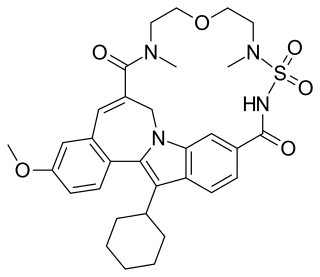
TMC-647055 is an experimental antiviral drug which was developed as a treatment for hepatitis C, and is in clinical trials as a combination treatment with ribavirin and simeprevir. It acts as a NS5b polymerase inhibitor.