Related Research Articles

Rotaviruses are the most common cause of diarrhoeal disease among infants and young children. Nearly every child in the world is infected with a rotavirus at least once by the age of five. Immunity develops with each infection, so subsequent infections are less severe. Adults are rarely affected. Rotavirus is a genus of double-stranded RNA viruses in the family Reoviridae. There are nine species of the genus, referred to as A, B, C, D, F, G, H, I and J. Rotavirus A is the most common species, and these rotaviruses cause more than 90% of rotavirus infections in humans.
A coiled coil is a structural motif in proteins in which 2–7 alpha-helices are coiled together like the strands of a rope. They have been found in roughly 5-10% of proteins and have a variety of functions. They are one of the most widespread motifs found in protein-protein interactions. To aid protein study, several tools have been developed to predict coiled-coils in protein structures. Many coiled coil-type proteins are involved in important biological functions, such as the regulation of gene expression — e.g., transcription factors. Notable examples are the oncoproteins c-Fos and c-Jun, as well as the muscle protein tropomyosin.

An enterotoxin is a protein exotoxin released by a microorganism that targets the intestines. They can be chromosomally or plasmid encoded. They are heat labile, of low molecular weight and water-soluble. Enterotoxins are frequently cytotoxic and kill cells by altering the apical membrane permeability of the mucosal (epithelial) cells of the intestinal wall. They are mostly pore-forming toxins, secreted by bacteria, that assemble to form pores in cell membranes. This causes the cells to die.

Tripartite motif-containing protein 5 also known as RING finger protein 88 is a protein that in humans is encoded by the TRIM5 gene. The alpha isoform of this protein, TRIM5α, is a retrovirus restriction factor, which mediates a species-specific early block to retrovirus infection.

A viroplasm, sometimes called "virus factory" or "virus inclusion", is an inclusion body in a cell where viral replication and assembly occurs. They may be thought of as viral factories in the cell. There are many viroplasms in one infected cell, where they appear dense to electron microscopy. Very little is understood about the mechanism of viroplasm formation.
The AB5 toxins are six-component protein complexes secreted by certain pathogenic bacteria known to cause human diseases such as cholera, dysentery, and hemolytic–uremic syndrome. One component is known as the A subunit, and the remaining five components are B subunits. All of these toxins share a similar structure and mechanism for entering targeted host cells. The B subunit is responsible for binding to receptors to open up a pathway for the A subunit to enter the cell. The A subunit is then able to use its catalytic machinery to take over the host cell's regular functions.

RoXaN also known as ZC3H7B, is a protein that in humans is encoded by the ZC3H7B gene. RoXaN is a protein that contains tetratricopeptide repeat and leucine-aspartate repeat as well as zinc finger domains. This protein also interacts with the rotavirus non-structural protein NSP3.
NSP1 (NS53), the product of rotavirus gene 5, is a nonstructural RNA-binding protein that contains a cysteine-rich region and is a component of early replication intermediates. RNA-folding predictions suggest that this region of the NSP1 mRNA can interact with itself, producing a stem-loop structure similar to that found near the 5'-terminus of the NSP1 mRNA.
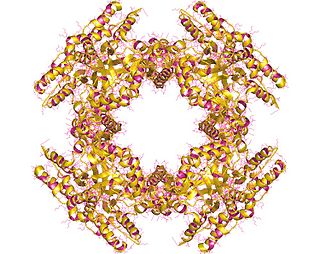
NSP2 (NS35), is one of five to six nonstructural proteins expressed by rotaviruses. The octameric NSP2 performs several key functions in the assembly of rotavirus particles. This nonstructural RNA-binding protein accumulates in cytoplasmic inclusions (viroplasms) and is required for genome replication. NSP2 is closely associated in vivo with the viral replicase. The non-structural protein NSP5 plays a role in the structure of viroplasms mediated by its interaction with NSP2.

Zinc finger and BTB domain-containing protein 16 is a protein that in humans is encoded by the ZBTB16 gene.

Homer protein homolog 1 or Homer1 is a neuronal protein that in humans is encoded by the HOMER1 gene. Other names are Vesl and PSD-Zip45.

Calcium-binding and coiled-coil domain-containing protein 2 is a protein that in humans is encoded by the CALCOCO2 gene.
Clostridium enterotoxins are toxins produced by Clostridium species. Clostridial species are one of the major causes of food poisoning/gastrointestinal illnesses. They are anaerobic, gram-positive, spore-forming rods that occur naturally in the soil. Among the family are: Clostridium botulinum, which produces one of the most potent toxins in existence; Clostridium tetani, causative agent of tetanus; and Clostridium perfringens, commonly found in wound infections and diarrhea cases.
Microbial toxins are toxins produced by micro-organisms, including bacteria, fungi, protozoa, dinoflagellates, and viruses. Many microbial toxins promote infection and disease by directly damaging host tissues and by disabling the immune system. Endotoxins most commonly refer to the lipopolysaccharide (LPS) or lipooligosaccharide (LOS) that are in the outer plasma membrane of Gram-negative bacteria. The botulinum toxin, which is primarily produced by Clostridium botulinum and less frequently by other Clostridium species, is the most toxic substance known in the world. However, microbial toxins also have important uses in medical science and research. Currently, new methods of detecting bacterial toxins are being developed to better isolate and understand these toxins. Potential applications of toxin research include combating microbial virulence, the development of novel anticancer drugs and other medicines, and the use of toxins as tools in neurobiology and cellular biology.

In the field of molecular biology, enterotoxin type B, also known as Staphylococcal enterotoxin B (SEB), is an enterotoxin produced by the gram-positive bacteria Staphylococcus aureus. It is a common cause of food poisoning, with severe diarrhea, nausea and intestinal cramping often starting within a few hours of ingestion. Being quite stable, the toxin may remain active even after the contaminating bacteria are killed. It can withstand boiling at 100 °C for a few minutes. Gastroenteritis occurs because SEB is a superantigen, causing the immune system to release a large amount of cytokines that lead to significant inflammation.

Ras and EF-hand domain-containing protein also known as Ras-related protein Rab-45 is a protein that in humans is encoded by the RASEF gene.
Mary K. Estes is an American virologist who is professor at Baylor College of Medicine in Houston, Texas. Her courses include microbiology, and virology; she is also the co-director of the Translational Biology and Molecular Medicine Graduate Program at Baylor College of Medicine. There are two main viruses that her research is based on, rotaviruses and noroviruses. The main goal of her research are to study how the viral proteins interact with the receptors of the intestinal cells; they are also looking into different ways to deliver virus-like particles to prevent these viruses from causing infections. Estes has achieved many awards and recognition in her time as a virologist. She is also a member of multiple foundations and professional societies.

Agnoprotein is a protein expressed by some members of the polyomavirus family from a gene called the agnogene. Polyomaviruses in which it occurs include two human polyomaviruses associated with disease, BK virus and JC virus, as well as the simian polyomavirus SV40.
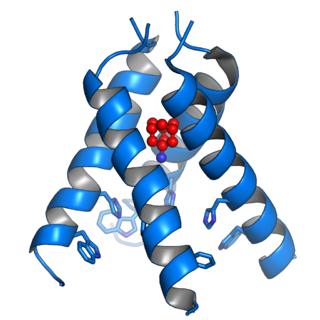
Viroporins are small and usually hydrophobic multifunctional viral proteins that modify cellular membranes, thereby facilitating virus release from infected cells. Viroporins are capable of assembling into oligomeric ion channels or pores in the host cell's membrane, rendering it more permeable and thus facilitating the exit of virions from the cell. Many viroporins also have additional effects on cellular metabolism and homeostasis mediated by protein-protein interactions with host cell proteins. Viroporins are not necessarily essential for viral replication, but do enhance growth rates. They are found in a variety of viral genomes but are particularly common in RNA viruses. Many viruses that cause human disease express viroporins. These viruses include hepatitis C virus, HIV-1, influenza A virus, poliovirus, respiratory syncytial virus, and SARS-CoV.

The envelope (E) protein is the smallest and least well-characterized of the four major structural proteins found in coronavirus virions. It is an integral membrane protein less than 110 amino acid residues long; in SARS-CoV-2, the causative agent of Covid-19, the E protein is 75 residues long. Although it is not necessarily essential for viral replication, absence of the E protein may produce abnormally assembled viral capsids or reduced replication. E is a multifunctional protein and, in addition to its role as a structural protein in the viral capsid, it is thought to be involved in viral assembly, likely functions as a viroporin, and is involved in viral pathogenesis.
References
- ↑ Dong Y, Zeng CQ, Ball JM, Estes MK, Morris AP (April 1997). "The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production". Proceedings of the National Academy of Sciences of the United States of America. 94 (8): 3960–5. Bibcode:1997PNAS...94.3960D. doi: 10.1073/pnas.94.8.3960 . PMC 20550 . PMID 9108087.
- ↑ Pham T, Perry JL, Dosey TL, Delcour AH, Hyser JM (March 2017). "The Rotavirus NSP4 Viroporin Domain is a Calcium-conducting Ion Channel". Scientific Reports. 7: 43487. Bibcode:2017NatSR...743487P. doi:10.1038/srep43487. PMC 5335360 . PMID 28256607.
- ↑ Gebert JT, Hyser J (May 2022). "Using Forward and Reverse Genetics to Understand Calcium Dysregulation in Enteric Viral Virulence". FASEB Journal. 36 (Suppl 1). doi: 10.1096/fasebj.2022.36.S1.R3214 . PMID 35557094. S2CID 248633853.
- ↑ Hu L, Crawford SE, Hyser JM, Estes MK, Prasad BV (August 2012). "Rotavirus non-structural proteins: structure and function". Current Opinion in Virology. 2 (4): 380–8. doi:10.1016/j.coviro.2012.06.003. PMC 3422752 . PMID 22789743.