Related Research Articles

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential, as a measurable and quantitative phenomenon, and identifiable chemical change, with either electrical potential as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving between electrodes via an electronically-conducting phase, separated by an ionically-conducting and electronically insulating electrolyte.
Ultrasound is sound waves with frequencies higher than the upper audible limit of human hearing. Ultrasound is not different from "normal" (audible) sound in its physical properties, except that humans cannot hear it. This limit varies from person to person and is approximately 20 kilohertz in healthy young adults. Ultrasound devices operate with frequencies from 20 kHz up to several gigahertz.

Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply.
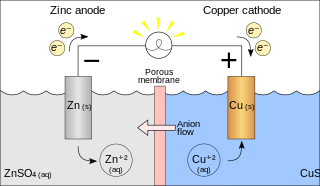
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge.

An electrolytic cell is an electrochemical cell that uses electrical energy to drive a non-spontaneous redox reaction. It is often used to decompose chemical compounds, in a process called electrolysis—the Greek word lysis means to break up. Important examples of electrolysis are the decomposition of water into hydrogen and oxygen, and bauxite into aluminium and other chemicals. Electroplating is done using an electrolytic cell. Electrolysis is a technique that uses a direct electric current (DC).
In electrochemistry, standard electrode potential (E°) is defined as The value of the standard emf of a cell in which molecular hydrogen under standard pressure is oxidized to solvated protons at the left-hand electrode.
Coulometry determines the amount of matter transformed during an electrolysis reaction by measuring the amount of electricity consumed or produced. It can be used for precision measurements of charge, and the amperes even used to have a coulometric definition. However, today coulometry is mainly used for analytical applications. Coulometry is a group of techniques in analytical chemistry. It is named after Charles-Augustin de Coulomb.

Sonication is the act of applying sound energy to agitate particles in a sample, for various purposes such as the extraction of multiple compounds from plants, microalgae and seaweeds. Ultrasonic frequencies (>20 kHz) are usually used, leading to the process also being known as ultrasonication or ultra-sonication.

Chronoamperometry is an electrochemical technique in which the potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells this decay is much slower than the charging decay-cells with no supporting electrolyte are notable exceptions. Most commonly a three electrode system is used. Since the current is integrated over relatively longer time intervals, chronoamperometry gives a better signal to noise ratio in comparison to other amperometric techniques.

Ultrasonic cleaning is a process that uses ultrasound to agitate a fluid. The ultrasound can be used with just water, but use of a solvent appropriate for the object to be cleaned and the type of soiling present enhances the effect. Cleaning normally lasts between three and six minutes, but can also exceed 20 minutes, depending on which object has to be cleaned.
In chemistry, the study of sonochemistry is concerned with understanding the effect of ultrasound in forming acoustic cavitation in liquids, resulting in the initiation or enhancement of the chemical activity in the solution. Therefore, the chemical effects of ultrasound do not come from a direct interaction of the ultrasonic sound wave with the molecules in the solution.
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency. In an electrolytic cell the existence of overpotential implies the cell requires more energy than thermodynamically expected to drive a reaction. In a galvanic cell the existence of overpotential means less energy is recovered than thermodynamics predicts. In each case the extra/missing energy is lost as heat. The quantity of overpotential is specific to each cell design and varies across cells and operational conditions, even for the same reaction. Overpotential is experimentally determined by measuring the potential at which a given current density is achieved.

A voltameter or coulometer is a scientific instrument used for measuring electric charge through electrolytic action. The SI unit of electric charge is the coulomb.
Platinum black is a fine powder of platinum with good catalytic properties. The name of platinum black is due to its black color. It is used in many ways; as a thin film electrode, a fuel cell membrane catalyst, or as a catalytic ignition of flammable gases for "self-lighting' gas lamps, ovens, and stove burners.
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are controlled and which are measured. The three main categories are potentiometry, coulometry, and voltammetry.
Bulk electrolysis is also known as potentiostatic coulometry or controlled potential coulometry. The experiment is a form of coulometry which generally employs a three electrode system controlled by a potentiostat. In the experiment the working electrode is held at a constant potential (volts) and current (amps) is monitored over time (seconds). In a properly run experiment an analyte is quantitatively converted from its original oxidation state to a new oxidation state, either reduced or oxidized. As the substrate is consumed, the current also decreases, approaching zero when the conversion nears completion.
In electrochemistry, polarization is a collective term for certain mechanical side-effects by which isolating barriers develop at the interface between electrode and electrolyte. These side-effects influence the reaction mechanisms, as well as the chemical kinetics of corrosion and metal deposition. In a reaction we can displace the bonding electrons by attacking reagents. The electronic displacement in turn may be due to certain effects, some of which are permanent, and the others are temporary. Those effects which are permanently operating in the molecule are known as polarization effects, and those effects which are brought into play by attacking reagent are known as polarisability effects.
Concentration polarization is a term used in the scientific fields of electrochemistry and membrane science.

Electrochemical quartz crystal microbalance (EQCM) is the combination of electrochemistry and quartz crystal microbalance, which was generated in the eighties. Typically, an EQCM device contains an electrochemical cells part and a QCM part. Two electrodes on both sides of the quartz crystal serve two purposes. Firstly, an alternating electric field is generated between the two electrodes for making up the oscillator. Secondly, the electrode contacting electrolyte is used as a working electrode (WE), together with a counter electrode (CE) and a reference electrode (RE), in the potentiostatic circuit constituting the electrochemistry cell. Thus, the working electrode of electrochemistry cell is the sensor of QCM.
In semiconductor physics, the flat band potential of a semiconductor defines the potential at which there is no depletion layer at the junction between a semiconductor and an electrolyte or p-n-junction. This is a consequence of the condition that the redox Fermi level of the electrolyte must be equal to the Fermi level of the semiconductor and therefore preventing any band bending of the conduction and valence band. An application of the flat band potential can be found in the determining the width of the space charge region in a semiconductor-electrolyte junction. Furthermore, it is used in the Mott-Schottky equation to determine the capacitance of the semiconductor-electrolyte junction and plays a role in the photocurrent of a photoelectrochemical cell. The value of the flat band potential depends on many factors, such as the material, Ph and crystal structure of the material
References
- ↑ Moriguchi, N. (1934)."The influence of supersonic waves on chemical phenomena. III–the influence on the concentration polarization".Nippon Kagaku Kaishi55: 749-750.
- ↑ Schmid, G., Ehret, L. (1937)."Beeinflussung der Metallpassivität durch Ultraschall".Berichte der Bunsengesellschaft für physikalische Chemie43(6): 408-415.
- ↑ Schmid G., Ehret L. (1937)."Beeinflussung der Elektrolytischen Abscheidungspotentiale von Gasen durch Ultraschall". Berichte der Bunsengesellschaft für physikalische Chemie43(8): 597–608.
- ↑ Kolb, J.,Nyborg, W.L. (1956)."Small‐Scale Acoustic Streaming in Liquids". The Journal of the Acoustical Society of America28(6): 1237-1242.
- ↑ Penn, R., Yeager, E., Hovorka, F. (1959)."Effect of Ultrasonic Waves on Concentration Gradients".The Journal of the Acoustical Society of America31(10): 1372-1376.
- ↑ Bard, A.J. (1963).“High Speed Controlled Potential Coulometry”.Analytical Chemistry35(9): 1125-1128.
- ↑ Hielscher - Ultrasound Technology (2017)."Hielscher".
- ↑ Pollet, B.G. (2012). Power Ultrasound in Electrochemistry: From Versatile Laboratory Tool to Engineering Solution. Wiley, ISBN 978-0-470-97424-7.
- ↑ Ozoemena, K.I., Chen, S. (2016). Nanomaterials for Fuel Cell Catalysis. Chapter 10 - 'Sonoelectrochemical Production of Fuel Cell Nanomaterials', Springer, ISBN 978-3-319-29930-3.
- ↑ Google Scholar - keyword: Sonoelectrochemistry.
- ↑ Mason, T.J., Lorimer, J.P., Walton, D.J. (1990)."Sonoelectrochemistry". Ultrasonics28(5): 333-337.
- ↑ Pollet, B.G. and Ashokkumar, M. (2019). Introduction to Ultrasound, Sonochemistry and Sonoelectrochemistry. Springer, ISBN 978-3-030-25862-7.