
In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.
In organic chemistry, the Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. It is one of the many oxidation reactions commonly referred to as 'activated DMSO' oxidations. The reaction is known for its mild character and wide tolerance of functional groups.

Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane.
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula R2C=N+=N−. The simplest example of a diazo compound is diazomethane, CH2N2. Diazo compounds should not be confused with azo compounds or with diazonium compounds.

The Curtius rearrangement, first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published.

Trimethylsilyldiazomethane is the organosilicon compound with the formula (CH3)3SiCHN2. It is classified as a diazo compound. Trimethylsilyldiazomethane is a commercially available reagent used in organic chemistry as a methylating agent and as a source of CH2 group. Its behavior is akin to the less convenient reagent diazomethane.
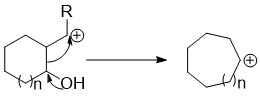
Ring expansion and ring contraction reactions expand or contract rings, usually in organic chemistry. The term usually refers to reactions involve making and breaking C-C bonds, Diverse mechanisms llead to these kinds of reactions.
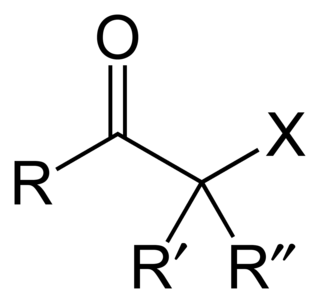
In organic chemistry, an α-halo ketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-Halo ketones are alkylating agents. Prominent α-halo ketones include phenacyl bromide and chloroacetone.

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.

In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroid insecticides and a number of quinolone antibiotics. However, the high ring strain present in cyclopropanes makes them challenging to produce and generally requires the use of highly reactive species, such as carbenes, ylids and carbanions. Many of the reactions proceed in a cheletropic manner.

Lead(IV) acetate or lead tetraacetate is an organometallic compound with chemical formula Pb(C2H3O2)4. It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis.

The Nierenstein reaction is an organic reaction describing the conversion of an acid chloride into a haloketone with diazomethane. It is an insertion reaction in that the methylene group from the diazomethane is inserted into the carbon-chlorine bond of the acid chloride.
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant unit, generally a methylene group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene units in saturated chain within the molecule. For example, the reaction of aldehydes or ketones with diazomethane or methoxymethylenetriphenylphosphine to give the next homologue in the series.
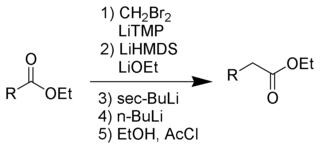
The Kowalski ester homologation is a chemical reaction for the homologation of esters.
Methanesulfonyl chloride is an organosulfur compound with the formula CH3SO2Cl. Using the organic pseudoelement symbol Ms for the methanesulfonyl group CH3SO2–, it is frequently abbreviated MsCl in reaction schemes or equations. It is a colourless liquid that dissolves in polar organic solvents but is reactive toward water, alcohols, and many amines. The simplest organic sulfonyl chloride, it is used to make methanesulfonates and to generate the elusive molecule sulfene.

Trifluoroacetic anhydride (TFAA) is the acid anhydride of trifluoroacetic acid. It is the perfluorinated derivative of acetic anhydride.
An insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:
The Buchner–Curtius–Schlotterbeck reaction is the reaction of aldehydes or ketones with aliphatic diazoalkanes to form homologated ketones. It was first described by Eduard Buchner and Theodor Curtius in 1885 and later by Fritz Schlotterbeck in 1907. Two German chemists also preceded Schlotterbeck in discovery of the reaction, Hans von Pechmann in 1895 and Viktor Meyer in 1905. The reaction has since been extended to the synthesis of β-keto esters from the condensation between aldehydes and diazo esters. The general reaction scheme is as follows:
The Danheiser benzannulation is a chemical reaction used in organic chemistry to generate highly substituted phenols in a single step. It is named after Rick L. Danheiser who developed the reaction.














