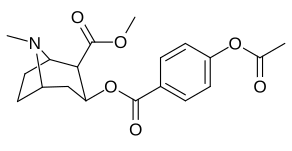
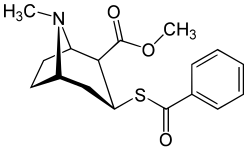
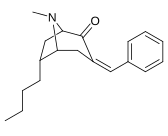
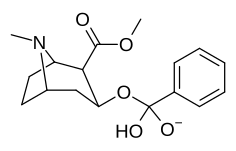
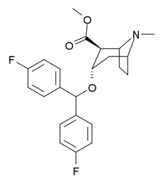
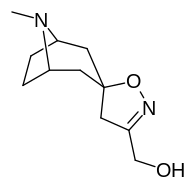
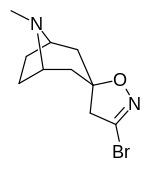
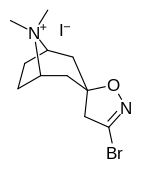
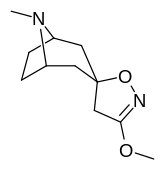
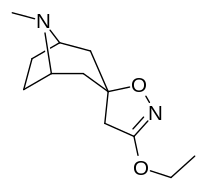
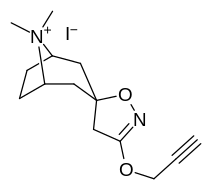
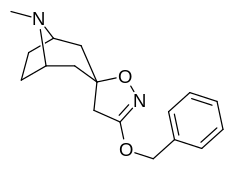
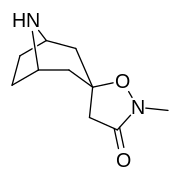
(–)-2-β-Carbomethoxy-3-β-(4-fluorophenyl)tropane is a stimulant drug used in scientific research. CFT is a phenyltropane based dopamine reuptake inhibitor and is structurally derived from cocaine. It is around 3-10x more potent than cocaine and lasts around 7 times longer based on animal studies. While the naphthalenedisulfonate salt is the most commonly used form in scientific research due to its high solubility in water, the free base and hydrochloride salts are known compounds and can also be produced. The tartrate is another salt form that is reported.

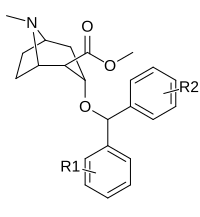
Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. This research has spanned beyond the last couple decades, and has picked up its pace in recent times, creating numerous phenyltropanes as research into cocaine analogues garners interest to treat addiction.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

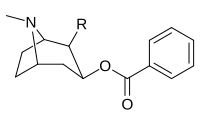
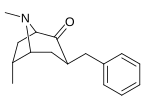
Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.
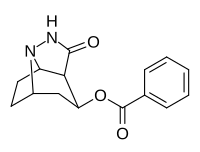
Mazindol is a stimulant drug which is used as an appetite suppressant. It was developed by Sandoz-Wander in the 1960s.

(–)-2β-Carboisopropoxy-3β-(4-iodophenyl)tropane is a stimulant drug used in scientific research, which was developed in the early 1990s. RTI-121 is a phenyltropane based, highly selective dopamine reuptake inhibitor and is derived from methylecgonidine. RTI-121 is a potent and long-lasting stimulant, producing stimulant effects for more than 10 hours after a single dose in mice which would limit its potential uses in humans, as it might have significant abuse potential if used outside a medical setting. However RTI-121 occupies the dopamine transporter more slowly than cocaine, and so might have lower abuse potential than cocaine itself.
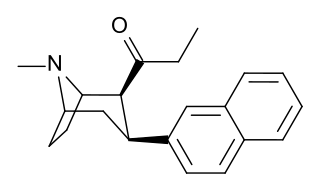
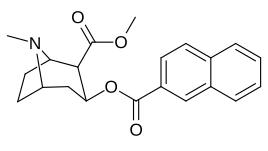
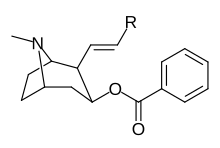
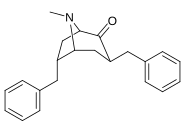
2β-Propanoyl-3β-(2-naphthyl)-tropane or WF-23 is a cocaine analogue. It is several hundred times more potent than cocaine at being a serotonin-norepinephrine-dopamine reuptake inhibitor.
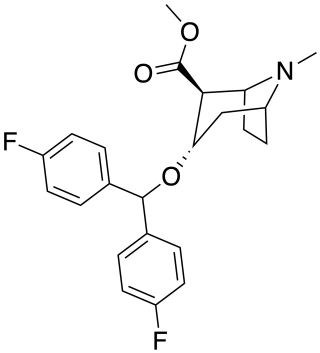
Difluoropine (O-620) is a stimulant drug synthesised from tropinone, which acts as a potent and selective dopamine reuptake inhibitor. Difluoropine is unique among the tropane-derived dopamine reuptake inhibitors in that the active stereoisomer is the (S) enantiomer rather than the (R) enantiomer, the opposite way round compared to natural cocaine. It is structurally related to benztropine and has similar anticholinergic and antihistamine effects in addition to its dopamine reuptake inhibitory action.
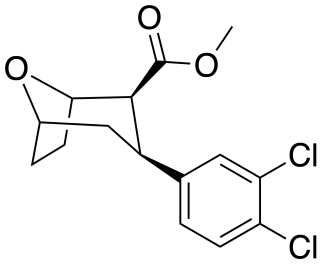
Tropoxane (O-1072) is an aryloxytropane derivative drug developed by Organix Inc., which acts as a stimulant and potent dopamine and serotonin reuptake inhibitor. It is an analogue of dichloropane where the amine nitrogen has been replaced by an oxygen ether link, demonstrating that the amine nitrogen is not required for DAT binding and reuptake inhibition.
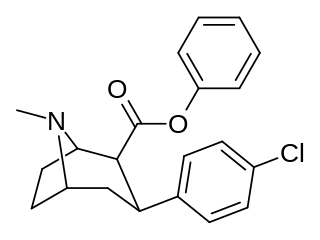
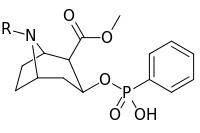
RTI(-4229)-113 is a stimulant drug which acts as a potent and fully selective dopamine reuptake inhibitor (DRI). It has been suggested as a possible substitute drug for the treatment of cocaine addiction. "RTI-113 has properties that make it an ideal medication for cocaine abusers, such as an equivalent efficacy, a higher potency, and a longer duration of action as compared to cocaine." Replacing the methyl ester in RTI-31 with a phenyl ester makes the resultant RTI-113 fully DAT specific. RTI-113 is a particularly relevant phenyltropane cocaine analog that has been tested on squirrel monkeys. RTI-113 has also been tested against cocaine in self-administration studies for DAT occupancy by PET on awake rhesus monkeys. The efficacy of cocaine analogs to elicit self-administration is closely related to the rate at which they are administered. Slower onset of action analogs are less likely to function as positive reinforcers than analogues that have a faster rate of onset.

RTI(-4229)-177 is a synthetic stimulant drug from the phenyltropane family, which acts as a DRI with micromolar affinity for the SERT. RTI-177 has an unusually long duration of action of 20 hours or more, substantially longer than the related compound RTI-336 from which it differs in molecular structure only by the absence of a p-methyl group.

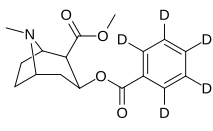
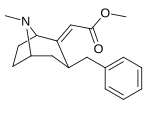
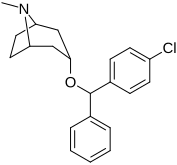
(–)-2β-Carbomethoxy-3β-(4'-chlorophenyl)tropane (RTI-4229-31) is a synthetic analog of cocaine that acts as a stimulant. Semi-synthesis of this compound is dependent upon the availability of cocaine starting material. According to the article, RTI-31 is 64 times the strength of cocaine in terms of its potency to elicit self-administration in monkeys. WIN 35428 was 6 times weaker than RTI-31, whereas RTI-51 was 2.6 times weaker than RTI-31.

(–)-2β-Carbomethoxy-3β-(4-bromophenyl)tropane is a semi-synthetic alkaloid in the phenyltropane group of psychostimulant compounds. First publicized in the 1990s, it has not been used enough to have gained a fully established profile. RTI-51 can be expected to have properties lying somewhere in between RTI-31 and RTI-55. It has a ratio of monoamine reuptake inhibition of dopamine > serotonin > norepinephrine which is an unusual balance of effects not produced by other commonly used compounds. It has been used in its 76Br radiolabelled form to map the distribution of dopamine transporters in the brain.

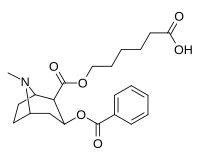
Salicylmethylecgonine, (2′-Hydroxycocaine) is a tropane derivative drug which is both a synthetic analogue and a possible active metabolite of cocaine. Its potency in vitro is around 10x that of cocaine, although it is only around three times more potent than cocaine when administered to mice Note however that the compound 2′-Acetoxycocaine would act as a prodrug to Salicylmethylecgonine in humans, and has a more efficient partition coefficient which would act as a delivery system and would circumvent this reason for a drop in potency. Salicylmethylecgonine also shows increased behavioral stimulation compared to cocaine similar to the phenyltropanes. The hydroxy branch renders the molecule a QSAR of a 10-fold increase over cocaine in its binding potency for the dopamine transporter & a 52-fold enhanced affinity for the norepinephrine transporter. It also has a reduced selectivity for the serotonin transporter though only due to its greater increase at NET binding; its SERT affinity being 4-fold increased compared to cocaine. However, in overall binding affinity it displaces ligands better across the board than cocaine in all monoamine categories.

RTI-229, also known as (–)-3β-(4-iodophenyl)tropane-2β-pyrrolidine carboxamide and RTI-4229-229, is a potent and long-lasting stimulant drug which was developed in the 1990s as part of a large group of related analogues from the phenyltropane family. With the combination of two potent dopamine transporter (DAT) binding motifs attached to the tropane ring, the p-iodophenyl group at the 3β-position and a pyrrolidine carboxamide at 2β, RTI-229 has extremely high selectivity for the dopamine transporter and is one of the most DAT-selective compounds in the RTI series.

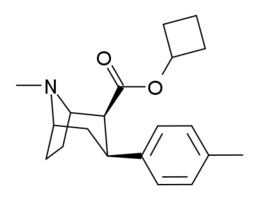
(–)-2β-Carbophenoxy-3β-(p-tolyl)tropane (RTI-4229-120) is a phenyltropane derivative which acts as a reasonably selective dopamine reuptake inhibitor, along with weaker inhibition of noradrenaline and serotonin reuptake. It has a reasonably fast rate of occupancy of dopamine transporters in the brain, though slower than that of cocaine itself. RTI-120 has a short duration of action, along with other p-methyl substituted phenyltropanes such as RTI-150, RTI-171 and RTI-199, giving it a more similar pharmacological profile to cocaine compared to longer acting analogues like RTI-121 and RTI-177.
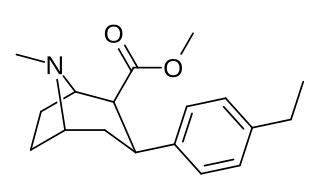
RTI-83 is a phenyltropane derivative which represents a rare example of an SDRI or serotonin-dopamine reuptake inhibitor, a drug which inhibits the reuptake of the neurotransmitters serotonin and dopamine, while having little or no effect on the reuptake of the related neurotransmitter noradrenaline. With a binding affinity (Ki) of 55 nM at DAT and 28.4 nM at SERT but only 4030 nM at NET, RTI-83 has reasonable selectivity for DAT/SERT over NET
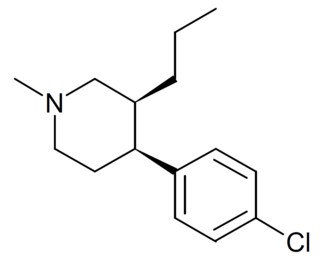
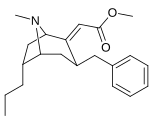
1-Methyl-3-propyl-4-(p-chlorophenyl)piperidine is a drug developed by a team led by Alan Kozikowski, which acts as a potent dopamine reuptake inhibitor, and was developed as a potential therapeutic agent for the treatment of cocaine addiction. As with related compounds such as nocaine, it is a structurally simplified derivative of related phenyltropane compounds. Its activity at the serotonin and noradrenaline transporters has not been published, though most related 4-phenylpiperidine derivatives are relatively selective for inhibiting dopamine reuptake over the other monoamine neurotransmitters. While several of its isomers are active, the (3S,4S)-enantiomer is by far the most potent. The rearranged structural isomer 2-[1-(4-chlorophenyl)butyl]piperidine is also a potent inhibitor of dopamine reuptake.