
Koninklijke Sint-Truidense Voetbalvereniging, commonly known as Sint-Truiden or STVV or by their nickname De Kanaries (Dutch pronunciation: [də kaːˈnaːris], is a Belgian professional football club located in the city of Sint-Truiden in Limburg. Sint-Truiden plays in the Belgian Pro League.

Saccharopinuria, also called saccharopinemia, saccharopine dehydrogenase deficiency or alpha-aminoadipic semialdehyde synthase deficiency, is a variant form of hyperlysinemia. It is caused by a partial deficiency of the enzyme saccharopine dehydrogenase, which plays a secondary role in the lysine metabolic pathway. Inheritance is thought to be autosomal recessive, but this cannot be established as individuals affected by saccharopinuria typically have only a 40% reduction in functional enzyme.

The Dutch units of measurement used today are those of the metric system. Before the 19th century, a wide variety of different weights and measures were used by the various Dutch towns and provinces. Despite the country's small size, there was a lack of uniformity. During the Dutch Golden Age, these weights and measures accompanied the Dutch to the farthest corners of their colonial empire, including South Africa, New Amsterdam and the Dutch East Indies. Units of weight included the pond, ons and last. There was also an apothecaries' system of weights. The mijl and roede were measurements of distance. Smaller distances were measured in units based on parts of the body – the el, the voet, the palm and the duim. Area was measured by the morgen, hont, roede and voet. Units of volume included the okshoofd, aam, anker, stoop, and mingel. At the start of the 19th century the Dutch adopted a unified metric system, but it was based on a modified version of the metric system, different from the system used today. In 1869, this was realigned with the international metric system. These old units of measurement have disappeared, but they remain a colourful legacy of the Netherlands' maritime and commercial importance and survive today in a number of Dutch sayings and expressions.

Succinic semialdehyde dehydrogenase deficiency (SSADHD) is a rare autosomal recessive disorder of the degradation pathway of the inhibitory neurotransmitter γ-aminobutyric acid, or GABA. The disorder has been identified in approximately 350 families, with a significant proportion being consanguineous families. The first case was identified in 1981 and published in a Dutch clinical chemistry journal that highlighted a number of neurological conditions such as delayed intellectual, motor, speech, and language as the most common manifestations. Later cases reported in the early 1990s began to show that hypotonia, hyporeflexia, seizures, and a nonprogressive ataxia were frequent clinical features as well.

Glutamate-1-semialdehyde is a molecule formed from by the reduction of tRNA bound glutamate, catalyzed by glutamyl-tRNA reductase. It is isomerized by glutamate-1-semialdehyde 2,1-aminomutase to give aminolevulinic acid in the biosynthesis of porphyrins, including heme and chlorophyll.

In enzymology, a succinate-semialdehyde dehydrogenase (SSADH) (EC 1.2.1.24) is an enzyme that catalyzes the chemical reaction
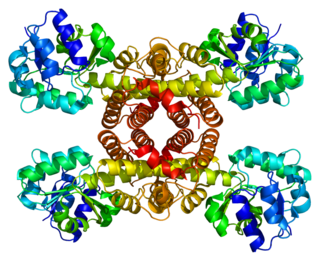
In enzymology, a 3-hydroxyisobutyrate dehydrogenase also known as β-hydroxyisobutyrate dehydrogenase or 3-hydroxyisobutyrate dehydrogenase, mitochondrial (HIBADH) is an enzyme that in humans is encoded by the HIBADH gene.

In enzymology, an aminomuconate-semialdehyde dehydrogenase (EC 1.2.1.32) is an enzyme that catalyzes the chemical reaction

In enzymology, an aspartate-semialdehyde dehydrogenase is an enzyme that is very important in the biosynthesis of amino acids in prokaryotes, fungi, and some higher plants. It forms an early branch point in the metabolic pathway forming lysine, methionine, leucine and isoleucine from aspartate. This pathway also produces diaminopimelate which plays an essential role in bacterial cell wall formation. There is particular interest in ASADH as disabling this enzyme proves fatal to the organism giving rise to the possibility of a new class of antibiotics, fungicides, and herbicides aimed at inhibiting it.

In enzymology, a methylmalonate-semialdehyde dehydrogenase (acylating) (EC 1.2.1.27) is an enzyme that catalyzes the chemical reaction

The enzyme aminocarboxymuconate-semialdehyde decarboxylase (EC 4.1.1.45) catalyzes the chemical reaction

In enzymology, 4-aminobutyrate transaminase, also called GABA transaminase or 4-aminobutyrate aminotransferase, or GABA-T, is an enzyme that catalyzes the chemical reaction:

Succinate-semialdehyde dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ALDH5A1 gene.

Methylmalonate-semialdehyde dehydrogenase [acylating], mitochondrial (MMSDH) is an enzyme that in humans is encoded by the ALDH6A1 gene.

2-Amino-3-carboxymuconic semialdehyde is an intermediate in the metabolism of tryptophan in the tryptophan-niacin catabolic pathway. Quinolinate is a neurotoxin formed nonenzymatically from 2-amino-3-carboxymuconic semialdehyde in mammalian tissues. 2-Amino-3-carboxymuconic semialdehyde is enzymatically converted to 2-aminomuconate via 2-aminomuconic semialdehyde.

Jacob Ferdinand Voet or Jakob Ferdinand Voet was a Flemish portrait painter. He had an international career that brought him to Italy and France, where he made portraits for an elite clientele. Voet is regarded as one of the best and most fashionable portrait painters of the High Baroque.

The gab operon is responsible for the conversion of γ-aminobutyrate (GABA) to succinate. The gab operon comprises three structural genes – gabD, gabT and gabP – that encode for a succinate semialdehyde dehydrogenase, GABA transaminase and a GABA permease respectively. There is a regulatory gene csiR, downstream of the operon, that codes for a putative transcriptional repressor and is activated when nitrogen is limiting.
2-hydroxy-4-carboxymuconate semialdehyde hemiacetal dehydrogenase (EC 1.1.1.312, 2-hydroxy-4-carboxymuconate 6-semialdehyde dehydrogenase, 4-carboxy-2-hydroxy-cis,cis-muconate-6-semialdehyde:NADP+ oxidoreductase, alpha-hydroxy-gamma-carboxymuconic epsilon-semialdehyde dehydrogenase, 4-carboxy-2-hydroxymuconate-6-semialdehyde dehydrogenase, LigC, ProD) is an enzyme with systematic name 4-carboxy-2-hydroxymuconate semialdehyde hemiacetal:NADP+ 2-oxidoreductase. This enzyme catalyses the following chemical reaction
Succinate-semialdehyde dehydrogenase (NADP+) (EC 1.2.1.79, succinic semialdehyde dehydrogenase (NADP+), succinyl semialdehyde dehydrogenase (NADP+), succinate semialdehyde:NADP+ oxidoreductase, NADP-dependent succinate-semialdehyde dehydrogenase, GabD) is an enzyme with systematic name succinate-semialdehyde:NADP+ oxidoreductase. This enzyme catalyses the following chemical reaction
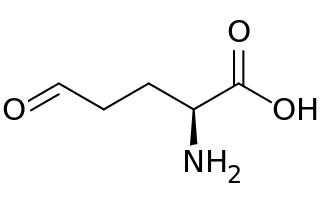
Glutamate-5-semialdehyde is a non-proteinogenic amino acid involved in both the biosynthesis and degradation of proline and arginine, as well as in the biosynthesis of antibiotics, such as carbapenems. It is synthesized by the reduction of glutamyl-5-phosphate by glutamate-5-semialdehyde dehydrogenase.


















