Related Research Articles

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

Redox is a type of chemical reaction in which the oxidation states of substrate change.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.

Geobacter is a genus of bacteria. Geobacter species are anaerobic respiration bacterial species which have capabilities that make them useful in bioremediation. Geobacter was found to be the first organism with the ability to oxidize organic compounds and metals, including iron, radioactive metals, and petroleum compounds into environmentally benign carbon dioxide while using iron oxide or other available metals as electron acceptors. Geobacter species are also found to be able to respire upon a graphite electrode. They have been found in anaerobic conditions in soils and aquatic sediment.
Microbial fuel cell (MFC) is a type of bioelectrochemical fuel cell system that generates electric current by diverting electrons produced from the microbial oxidation of reduced compounds on the anode to oxidized compounds such as oxygen on the cathode through an external electrical circuit. MFCs can be grouped into two general categories: mediated and unmediated. The first MFCs, demonstrated in the early 20th century, used a mediator: a chemical that transfers electrons from the bacteria in the cell to the anode. Unmediated MFCs emerged in the 1970s; in this type of MFC the bacteria typically have electrochemically active redox proteins such as cytochromes on their outer membrane that can transfer electrons directly to the anode. In the 21st century MFCs have started to find commercial use in wastewater treatment.
Electrosynthesis in chemistry is the synthesis of chemical compounds in an electrochemical cell. Compared to ordinary redox reaction, electrosynthesis sometimes offers improved selectivity and yields. Electrosynthesis is actively studied as a science and also has industrial applications. Electrooxidation has potential for wastewater treatment as well.
An enzymatic biofuel cell is a specific type of fuel cell that uses enzymes as a catalyst to oxidize its fuel, rather than precious metals. Enzymatic biofuel cells, while currently confined to research facilities, are widely prized for the promise they hold in terms of their relatively inexpensive components and fuels, as well as a potential power source for bionic implants.

Shewanella oneidensis is a bacterium notable for its ability to reduce metal ions and live in environments with or without oxygen. This proteobacterium was first isolated from Lake Oneida, NY in 1988, hence its name.
Many microorganisms can naturally grow together on surfaces to form complex aggregations called biofilms. Much research has been done on methods to remove biofilms in clinical and food manufacturing processes, but biofilms are also used for constructive purposes in a variety of industries. One distinctive characteristic of biofilm formation is that microorganisms within biofilms are often much tougher and more resistant to environmental stress compared to individual microorganisms. The cells are stationary and are able to adapt to adverse environments. This phenomenon of enhanced resistance can be beneficial in industrial chemical production where microorganisms within biofilms may tolerate higher chemical concentration and act as robust biorefineries for various products. These microbes have also been used in bioremediation to remove contaminants from freshwater and wastewater. More novel uses of biofilms include generating electricity using microbial fuel cells. Challenges to scaling up this technology include cost, controlling the growth of biofilms, and membrane fouling.
Electromethanogenesis is a form of electrofuel production where methane is produced by direct biological conversion of electrical current and carbon dioxide.

Bacterial nanowires are electrically conductive appendages produced by a number of bacteria most notably from the Geobacter and Shewanella genera. Conductive nanowires have also been confirmed in the oxygenic cyanobacterium Synechocystis PCC6803 and a thermophilic, methanogenic coculture consisting of Pelotomaculum thermopropionicum and Methanothermobacter thermoautotrophicus. From physiological and functional perspectives, bacterial nanowires are diverse. The precise role microbial nanowires play in their biological systems has not been fully realized, but several proposed functions exist. Outside of a naturally occurring environment, bacterial nanowires have shown potential to be useful in several fields, notably the bioenergy and bioremediation industries.
Rhodopseudomonas palustris is a rod-shaped, Gram-negative purple nonsulfur bacterium, notable for its ability to switch between four different modes of metabolism.

A microbial electrolysis cell (MEC) is a technology related to Microbial fuel cells (MFC). Whilst MFCs produce an electric current from the microbial decomposition of organic compounds, MECs partially reverse the process to generate hydrogen or methane from organic material by applying an electric current. The electric current would ideally be produced by a renewable source of power. The hydrogen or methane produced can be used to produce electricity by means of an additional PEM fuel cell or internal combustion engine.
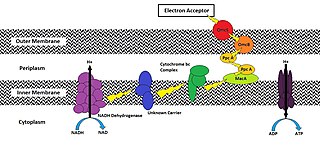
An exoelectrogen normally refers to a microorganism that has the ability to transfer electrons extracellularly. While exoelectrogen is the predominant name, other terms have been used: electrochemically active bacteria, anode respiring bacteria, and electricigens. Electrons exocytosed in this fashion are produced following ATP production using an electron transport chain (ETC) during oxidative phosphorylation. Conventional cellular respiration requires a final electron acceptor to receive these electrons. Cells that use molecular oxygen (O2) as their final electron acceptor are described as using aerobic respiration, while cells that use other soluble compounds as their final electron acceptor are described as using anaerobic respiration. However, the final electron acceptor of an exoelectrogen is found extracellularly and can be a strong oxidizing agent in aqueous solution or a solid conductor/electron acceptor. Two commonly observed acceptors are iron compounds (specifically Fe(III) oxides) and manganese compounds (specifically Mn(III/IV) oxides). As oxygen is a strong oxidizer, cells are able to do this strictly in the absence of oxygen.
A Bioelectrochemical reactor is a type of bioreactor where bioelectrochemical processes are used to degrade/produce organic materials using microorganisms. This bioreactor has two compartments: The anode, where the oxidation reaction takes place; And the cathode, where the reduction occurs. At these sites, electrons are passed to and from microbes to power reduction of protons, breakdown of organic waste, or other desired processes. They are used in microbial electrosynthesis, environmental remediation, and electrochemical energy conversion. Examples of bioelectrochemical reactors include microbial electrolysis cells, microbial fuel cells, enzymatic biofuel cells, electrolysis cells, microbial electrosynthesis cells, and biobatteries.
Microbial electrosynthesis (MES) is a form of microbial electrocatalysis in which electrons are supplied to living microorganisms via a cathode in an electrochemical cell by applying an electric current. The electrons are then used by the microorganisms to reduce carbon dioxide to yield industrially relevant products. The electric current would ideally be produced by a renewable source of power. This process is the opposite to that employed in a microbial fuel cell, in which microorganisms transfer electrons from the oxidation of compounds to an anode to generate an electric current.

Electrofuels, also known as e-fuels or synthetic fuels, are a type of drop-in replacement fuel. They are manufactured using captured carbon dioxide or carbon monoxide, together with hydrogen obtained from sustainable electricity sources such as wind, solar and nuclear power.
Geopsychrobacter electrodiphilus is a species of bacteria, the type species of its genus. It is a psychrotolerant member of its family, capable of attaching to the anodes of sediment fuel cells and harvesting electricity by oxidation of organic compounds to carbon dioxide and transferring the electrons to the anode.
Biological photovoltaics, also called biophotovoltaics or BPV, is an energy-generating technology which uses oxygenic photoautotrophic organisms, or fractions thereof, to harvest light energy and produce electrical power. Biological photovoltaic devices are a type of biological electrochemical system, or microbial fuel cell, and are sometimes also called photo-microbial fuel cells or “living solar cells”. In a biological photovoltaic system, electrons generated by photolysis of water are transferred to an anode. A relatively high-potential reaction takes place at the cathode, and the resulting potential difference drives current through an external circuit to do useful work. It is hoped that using a living organism as the light harvesting material, will make biological photovoltaics a cost-effective alternative to synthetic light-energy-transduction technologies such as silicon-based photovoltaics.
A microbial desalination cell (MDC) is a biological electrochemical system that implements the use of electro-active bacteria to power desalination of water in situ, resourcing the natural anode and cathode gradient of the electro-active bacteria and thus creating an internal supercapacitor. Available water supply has become a worldwide endemic as only .3% of the earth's water supply is usable for human consumption, while over 99% is sequestered by oceans, glaciers, brackish waters, and biomass. Current applications in electrocoagulation, such as microbial desalination cells, are able to desalinate and sterilize formerly unavailable water to render it suitable for safe water supply. Microbial desalination cells stem from microbial fuel cells, deviating by no longer requiring the use of a mediator and instead relying on the charged components of the internal sludge to power the desalination process. Microbial desalination cells therefore do not require additional bacteria to mediate the catabolism of the substrate during biofilm oxidation on the anodic side of the capacitor. MDCs and other bio-electrical systems are favored over reverse osmosis, nanofiltration and other desalination systems due to lower costs, energy and environmental impacts associated with bio-electrical systems.
References
- ↑ Logan, B.E., et al., Electroactive microorganisms in bioelectrochemical systems. Nature Reviews Microbiology, 2019. 17(5): p. 307-319.
- ↑ Nevin, K.P., et al., Microbial Electrosynthesis: Feeding Microbes Electricity To Convert Carbon Dioxide and Water to Multicarbon Extracellular Organic Compounds. mBio, 2010. 1(2): p. e00103-10.
- ↑ Logan, B.E., Exoelectrogenic bacteria that power microbial fuel cells. Nature Reviews Microbiology, 2009. 7(5): p. 375-381.
- ↑ Rozendal, R.A., et al., Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system. Electrochemistry Communications, 2009.11(9): p. 1752-175
- ↑ Harnisch, F. and U. Schroder, From MFC to MXC: chemical and biological cathodes and their potential for microbial bioelectrochemical systems. Chem Soc Rev, 2010. 39(11): p. 4433-48.
- ↑ Kumar, P., et al., Electro-Fermentation in Aid of Bioenergy and Biopolymers. Energies, 2018. 11(2): p. 343-343.
- ↑ Bajracharya, S., et al., An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renewable Energy, 2016. 98: p. 153-170.
- ↑ Potter, M.C. and A.D. Waller, Electrical effects accompanying the decomposition of organic compounds. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character, 1911. 84(571): p. 260-276.
- ↑ Cohen, B., The bacterial culture as an electrical half-cell. J. Bacteriol, 1931. 21(1): p. 18-19.
- ↑ Myers, C.R. and K.H. Nealson, Bacterial Manganese Reduction and Growth with Manganese Oxide as the Sole Electron-Acceptor. Science, 1988. 240(4857): p. 1319-1321.
- ↑ Kim, H.J., et al., A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme and Microbial Technology, 2002. 30(2): p. 145-152.
- ↑ Liu, H., R. Ramnarayanan, and B.E. Logan, Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol, 2004. 38(7): p. 2281-5.
- ↑ Rabaey, K., et al., A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. Biotechnology Letters, 2003. 25(18): p. 1531-1535.
- ↑ Logan, B.E., et al., Microbial Fuel Cells: Methodology and Technology. Environmental Science & Technology, 2006. 40(17): p. 5181-5192.
- ↑ Gregory, K.B., D.R. Bond, and D.R. Lovley, Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol, 2004. 6(6): p. 596-604.
- ↑ Nevin, K.P., et al., Microbial Electrosynthesis: Feeding Microbes Electricity To Convert Carbon Dioxide and Water to Multicarbon Extracellular Organic Compounds. Mbio, 2010. 1(2).
- ↑ Logan, B.E., et al., Electroactive microorganisms in bioelectrochemical systems. Nat Rev Microbiol, 2019. 17(5): p. 307-319.
- ↑ Izadi, P., et al., Parameters influencing the development of highly conductive and efficient biofilm during microbial electrosynthesis: the importance of applied potential and inorganic carbon source. NPJ Biofilms Microbiomes, 2020. 6(1): p. 40.
- ↑ Bian, B., et al., Resistance assessment of microbial electrosynthesis for biochemical production to changes in delivery methods and CO2 flow rates. Bioresour Technol, 2021. 319: p. 124177.
- ↑ Alqahtani, M.F., et al., Enrichment of salt-tolerant CO2-fixing communities in microbial electrosynthesis systems using porous ceramic hollow tube wrapped with carbon cloth as cathode and for CO2 supply. Sci Total Environ, 2021. 766: p. 142668.
- ↑ Jourdin, L., et al., Bringing High-Rate, CO2-Based Microbial Electrosynthesis Closer to Practical Implementation through Improved Electrode Design and Operating Conditions. Environ Sci Technol, 2016. 50(4): p. 1982-9.
- ↑ Zhou, H., et al., Optimization of a newly developed electromethanogenesis for the highest record of methane production. J Hazard Mater, 2021. 407: p. 124363.
- ↑ Srikanth, S., et al., Electro-biocatalytic conversion of carbon dioxide to alcohols using gas diffusion electrode. Bioresour Technol, 2018. 265: p. 45-51.
- ↑ Rosenbaum, M., et al., Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresour Technol, 2011. 102(1): p. 324-33.
- ↑ Myers, C.R. and J.M. Myers, Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bacteriol, 1992. 174(11): p. 3429-38.
- ↑ Tang, H.Y., et al., Iron Corrosion via Direct Metal-Microbe Electron Transfer. mBio, 2019. 10(3).
- ↑ Okamoto, A., et al., Bound Flavin Model Suggests Similar Electron-Transfer Mechanisms in Shewanella and Geobacter. ChemElectroChem, 2014. 1(11): p. 1808-1812.
- ↑ Newman, D.K. and R. Kolter, A role for excreted quinones in extracellular electron transfer. Nature, 2000. 405(6782): p. 94-7.
- ↑ Derek R. Lovley, J.D.C., Elizabeth L. Blunt-Harris, Elizabeth J. P. Phillips, Joan C. Woodward Humic substances as electron acceptors for microbial respiration. Nature, 1996(382): p. 445–448.
- ↑ You, L.X., et al., Flavins mediate extracellular electron transfer in Gram-positive Bacillus megaterium strain LLD-1. Bioelectrochemistry, 2018. 119: p. 196-202.
- ↑ Holmes, D.E., D.R. Bond, and D.R. Lovley, Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes. Applied and environmental microbiology, 2004. 70(2): p. 1234-1237.
- ↑ Rabaey, K., et al., Microbial Phenazine Production Enhances Electron Transfer in Biofuel Cells. Environmental Science & Technology, 2005. 39(9): p. 3401-3408.
- ↑ Rabaey, K., et al., Biofuel Cells Select for Microbial Consortia That Self-Mediate Electron Transfer. Applied and Environmental Microbiology, 2004. 70(9): p. 5373.
- ↑ Marsili, E., et al., <em>Shewanella</em> secretes flavins that mediate extracellular electron transfer. Proceedings of the National Academy of Sciences, 2008. 105(10): p. 3968.
- ↑ von Canstein, H., et al., Secretion of Flavins by <em>Shewanella</em> Species and Their Role in Extracellular Electron Transfer. Applied and Environmental Microbiology, 2008. 74(3): p. 615.
- ↑ Okamoto, A., et al., Bound Flavin Model Suggests Similar Electron-Transfer Mechanisms in Shewanella and Geobacter. ChemElectroChem, 2014. 1(11): p. 1808-1812
- ↑ Okamoto, A., et al., Proton Transport in the Outer-Membrane Flavocytochrome Complex Limits the Rate of Extracellular Electron Transport. Angewandte Chemie International Edition, 2017. 56(31): p. 9082-9086.
- ↑ Bond, D.R. and D.R. Lovley, Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol, 2003. 69(3): p. 1548-55.
- ↑ Reguera, G., et al., Extracellular electron transfer via microbial nanowires. Nature, 2005. 435(7045): p. 1098-1101.
- ↑ Logan, B.E. and J.M. Regan, Electricity-producing bacterial communities in microbial fuel cells. Trends in Microbiology, 2006. 14(12): p. 512-518.
- ↑ Osman, M.H., A.A. Shah, and F.C. Walsh, Recent progress and continuing challenges in bio-fuel cells. Part I: Enzymatic cells. Biosensors and Bioelectronics, 2011. 26(7): p. 3087-3102.
- ↑ Hyung-Joo, K.I.M., H. Moon-Sik, and C. In-Seop, A Microbial Fuel Cell Type Lactate Biosensor Using a Metal - Reducing Bacterium , Shewanella putrefaciens. Journal of Microbiology and Biotechnology, 1999. 9(3): p. 365-367.
- ↑ A. Oji, C.C.O.a.M.K.O., Fundamentals and Field Application of Microbial Fuel cells (MFCs). Euro. J. Appl. Eng. Sci. Res, 2012. 1(4): p. 185-189.
- ↑ Bond, D.R., et al., Electrode-reducing microorganisms that harvest energy from marine sediments. Science, 2002. 295(5554): p. 483-5.
- ↑ Tender, L.M., et al., Harnessing microbially generated power on the seafloor. Nature Biotechnology, 2002. 20(8): p. 821-825.
- ↑ Nancharaiah, Y.V., S. Venkata Mohan, and P.N.L. Lens, Recent advances in nutrient removal and recovery in biological and bioelectrochemical systems. Bioresour Technol, 2016. 215: p. 173-185.
- ↑ Cusick, R.D. and B.E. Logan, Phosphate recovery as struvite within a single chamber microbial electrolysis cell. Bioresource Technology, 2012. 107: p. 110-115.
- ↑ Zhang, F., J. Li, and Z. He, A new method for nutrients removal and recovery from wastewater using a bioelectrochemical system. Bioresource Technology, 2014. 166: p. 630-634.
- ↑ Mohan, S.V. and K. Chandrasekhar, Self-induced bio-potential and graphite electron accepting conditions enhances petroleum sludge degradation in bio-electrochemical system with simultaneous power generation. Bioresource Technology, 2011. 102(20): p. 9532-9541.
- ↑ Morris, J.M., et al., Microbial fuel cell in enhancing anaerobic biodegradation of diesel. Chemical Engineering Journal, 2009. 146(2): p. 161-167.
- ↑ Lovley, D.R. and K.P. Nevin, A shift in the current: new applications and concepts for microbe-electrode electron exchange. Curr Opin Biotechnol, 2011. 22(3): p. 441-8.
- ↑ Chandrasekhar, K. and S. Venkata Mohan, Bio-electrochemical remediation of real field petroleum sludge as an electron donor with simultaneous power generation facilitates biotransformation of PAH: Effect of substrate concentration. Bioresource Technology, 2012. 110: p. 517-525.
- ↑ Aulenta, F., et al., Trichloroethene dechlorination and H2 evolution are alternative biological pathways of electric charge utilization by a dechlorinating culture in a bioelectrochemical system. Environ Sci Technol, 2008. 42(16): p. 6185-90.
- ↑ Ceballos-Escalera, A., et al., Electro-bioremediation of nitrate and arsenite polluted groundwater. Water Res, 2021. 190: p. 116748.
- ↑ Zhao, H., et al., Performance of Denitrifying Microbial Fuel Cell with Biocathode over Nitrite. Frontiers in Microbiology, 2016. 7(344).
- ↑ Virdis, B., et al., Electron fluxes in a microbial fuel cell performing carbon and nitrogen removal. Environ Sci Technol, 2009. 43(13): p. 5144-9.
- ↑ Butler, C.S., et al., Bioelectrochemical Perchlorate Reduction in a Microbial Fuel Cell. Environmental Science & Technology, 2010. 44(12): p. 4685-4691.
- ↑ Wang, G., L.P. Huang, and Y.F. Zhang, Cathodic reduction of hexavalent chromium [Cr(VI)] coupled with electricity generation in microbial fuel cells. Biotechnology Letters, 2008. 30(11): p. 1959-1966.
- ↑ Gregory, K.B. and D.R. Lovley, Remediation and recovery of uranium from contaminated subsurface environments with electrodes. Environmental Science & Technology, 2005. 39(22): p. 8943-8947.
- ↑ Dominguez-Garay, A., et al., Bioelectroventing: an electrochemical-assisted bioremediation strategy for cleaning-up atrazine-polluted soils. Microb Biotechnol, 2018. 11(1): p. 50-62.
- ↑ Rodrigo Quejigo, J., et al., Stimulating soil microorganisms for mineralizing the herbicide isoproturon by means of microbial electroremediating cells. Microb Biotechnol, 2016. 9(3): p. 369-80.
- ↑ Rabaey, K. and R.A. Rozendal, Microbial electrosynthesis — revisiting the electrical route for microbial production. Nature Reviews Microbiology, 2010. 8(10): p. 706-716.
- ↑ Irfan, M., et al., Direct microbial transformation of carbon dioxide to value-added chemicals: A comprehensive analysis and application potentials. Bioresource Technology, 2019. 288: p. 121401.
- ↑ Miran, W., et al., Chlorinated phenol treatment and in situ hydrogen peroxide production in a sulfate-reducing bacteria enriched bioelectrochemical system. Water Research, 2017. 117: p. 198-206.
- ↑ Marshall, C.W., et al., Long-term Operation of Microbial Electrosynthesis Systems Improves Acetate Production by Autotrophic Microbiomes. Environmental Science & Technology, 2013. 47(11): p. 6023-6029.
- ↑ van Eerten-Jansen, M.C.A.A., et al., Analysis of the mechanisms of bioelectrochemical methane production by mixed cultures. Journal of Chemical Technology & Biotechnology, 2015. 90(5): p. 963-970.
- ↑ Bajracharya, S., et al., Carbon dioxide reduction by mixed and pure cultures in microbial electrosynthesis using an assembly of graphite felt and stainless steel as a cathode. Bioresource Technology, 2015. 195: p. 14-24.
- ↑ Kumar, G., et al., A review on bio-electrochemical systems (BESs) for the syngas and value added biochemicals production. Chemosphere, 2017. 177: p. 84-92.
- ↑ Kumar, A., et al., The ins and outs of microorganism–electrode electron transfer reactions. Nature Reviews Chemistry, 2017. 1(3).
- ↑ Philips, J., Extracellular Electron Uptake by Acetogenic Bacteria: Does H2 Consumption Favor the H2 Evolution Reaction on a Cathode or Metallic Iron? Front Microbiol, 2019. 10: p. 2997.
- ↑ Philips, J., Extracellular Electron Uptake by Acetogenic Bacteria: Does H2 Consumption Favor the H2 Evolution Reaction on a Cathode or Metallic Iron? Front Microbiol, 2019. 10: p. 2997.
- ↑ Dessi, P., et al., Carboxylic acids production and electrosynthetic microbial community evolution under different CO2 feeding regimens. Bioelectrochemistry, 2021. 137: p. 107686.
- ↑ Dessì, P., et al., Microbial electrosynthesis: Towards sustainable biorefineries for production of green chemicals from CO(2) emissions. Biotechnol Adv, 2021. 46: p. 107675.
- ↑ van Eerten-Jansen, M.C.A.A., et al., Analysis of the mechanisms of bioelectrochemical methane production by mixed cultures. Journal of Chemical Technology & Biotechnology, 2015. 90(5): p. 963-970.
- ↑ Steinbusch, K.J.J., et al., Bioelectrochemical Ethanol Production through Mediated Acetate Reduction by Mixed Cultures. Environmental Science & Technology, 2010. 44(1): p. 513-517.
- ↑ Raes, S.M.T., et al., Continuous Long-Term Bioelectrochemical Chain Elongation to Butyrate. ChemElectroChem, 2017. 4(2): p. 386-395.
- ↑ Kim, I.S., et al., Microbial Fuel Cells: Recent Advances, Bacterial Communities and Application Beyond Electricity Generation. Environmental Engineering Research, 2008. 13(2): p. 51-65.
- ↑ Logan, B.E., MECs for Hydrogen Production, in Microbial Fuel Cells. 2007, Wiley, 2008. p. 125-145.
- ↑ Rabaey, K. and W. Verstraete, Microbial fuel cells: novel biotechnology for energy generation. Trends in Biotechnology, 2005. 23(6): p. 291-298.
- ↑ Du, Z., H. Li, and T. Gu, A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnology Advances, 2007. 25(5): p. 464-482.
- ↑ Nelabhotla, A.B.T. and C. Dinamarca, Electrochemically mediated CO2 reduction for bio-methane production: a review. Reviews in Environmental Science and Bio/Technology, 2018. 17(3): p. 531-551.
- ↑ Sánchez, O.G., et al., Recent advances in industrial CO2 electroreduction. Current Opinion in Green and Sustainable Chemistry, 2019. 16: p. 47-56.
- ↑ Angenent, L.T., et al., Integrating electrochemical, biological, physical, and thermochemical process units to expand the applicability of anaerobic digestion. Bioresource Technology, 2018. 247: p. 1085-1094.
- ↑ Kim, Y. and B.E. Logan, Microbial desalination cells for energy production and desalination. Desalination, 2013. 308: p. 122-130.
- ↑ Cao, X., et al., A New Method for Water Desalination Using Microbial Desalination Cells. Environmental Science & Technology, 2009. 43(18): p. 7148-7152.
- ↑ Di Lorenzo, M., et al., A single-chamber microbial fuel cell as a biosensor for wastewaters. Water Research, 2009. 43(13): p. 3145-3154.
- ↑ Kim, B.H., et al., Novel BOD (biological oxygen demand) sensor using mediator-less microbial fuel cell. Biotechnology Letters, 2003. 25(7): p. 541-545.
- ↑ Chang, I.S., et al., Improvement of a microbial fuel cell performance as a BOD sensor using respiratory inhibitors. Biosensors and Bioelectronics, 2005. 20(9): p. 1856-1859.
- ↑ Lovley, D.R., Microbial fuel cells: novel microbial physiologies and engineering approaches. Current Opinion in Biotechnology, 2006. 17(3): p. 327-332.
- ↑ Chang, I.S., et al., Continuous determination of biochemical oxygen demand using microbial fuel cell type biosensor. Biosensors and Bioelectronics, 2004. 19(6): p. 607-613.
- ↑ Meyer, R.L., L.H. Larsen, and N.P. Revsbech, Microscale Biosensor for Measurement of Volatile Fatty Acids in Anoxic Environments. Applied and Environmental Microbiology, 2002. 68(3): p. 1204.
- ↑ Wrighton, K. and J. Coates, Microbial Fuel Cells: Plug-in and Power-on Microbiology. Microbe Magazine, 2009. 4: p. 281-287.
- ↑ Shantaram, A., et al., Wireless Sensors Powered by Microbial Fuel Cells. Environmental Science & Technology, 2005. 39(13): p. 5037-5042.
- ↑ Miran, W., D. Naradasu, and A. Okamoto, Pathogens electrogenicity as a tool for in-situ metabolic activity monitoring and drug assessment in biofilms. iScience, 2021. 24(2): p. 102068.
- ↑ Naradasu, D., et al., Microbial current production from Streptococcus mutans correlates with biofilm metabolic activity. Biosens Bioelectron, 2020. 162: p. 112236.
- ↑ 94.Miran, W., et al., Current Production Capability of Drug-Resistant Pathogen Enables Its Rapid Label-Free Detection Applicable to Wastewater-Based Epidemiology. Microorganisms, 2022. 10(2).