| Periaqueductal gray | |
|---|---|
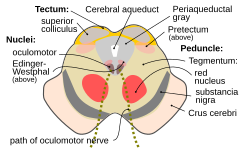 Section through superior colliculus showing path of oculomotor nerve. Periaqueductal gray is the gray area just peripheral to the cerebral aqueduct. | |
 Transverse section through mid-brain.
| |
| Details | |
| Identifiers | |
| Latin | substantia grisea centralis |
| MeSH | D010487 |
| NeuroNames | 1584 |
| NeuroLex ID | birnlex_973 |
| TA98 | A14.1.06.321 |
| TA2 | 5909 |
| FMA | 83134 |
| Anatomical terms of neuroanatomy | |
The periaqueductal gray (PAG), also known as the central gray, is a brain region that plays a critical role in autonomic function, motivated behavior and behavioural responses to threatening stimuli. [1] [2] PAG is also the primary control center for descending pain modulation. It has enkephalin-producing cells that suppress pain.
Contents
- Role in analgesia
- Role in defensive behavior
- Role in reproductive behavior
- Role in maternal behavior
- Additional images
- See also
- References
- External links
The periaqueductal gray is the gray matter located around the cerebral aqueduct within the tegmentum of the midbrain. It projects to the nucleus raphe magnus, and also contains descending autonomic tracts. The ascending pain and temperature fibers of the spinothalamic tract send information to the PAG via the spinomesencephalic pathway (so-named because the fibers originate in the spine and terminate in the PAG, in the mesencephalon or midbrain).
This region has been used as the target for brain-stimulating implants in patients with chronic pain.



