Orexin, also known as hypocretin, is a neuropeptide that regulates arousal, wakefulness, and appetite. The most common form of narcolepsy, type 1, in which the individual experiences brief losses of muscle tone, is caused by a lack of orexin in the brain due to destruction of the cells that produce it. It exists in the forms of orexin-A and orexin-B.
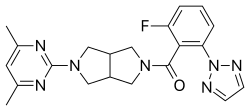
SB-649868 is a dual orexin receptor antagonist that was being developed by GlaxoSmithKline as a treatment for insomnia.
The orexin receptor (also referred to as the hypocretin receptor) is a G-protein-coupled receptor that binds the neuropeptide orexin. There are two variants, OX1 and OX2, each encoded by a different gene (HCRTR1, HCRTR2).

Orexin receptor type 1 (Ox1R or OX1), also known as hypocretin receptor type 1 (HcrtR1), is a protein that in humans is encoded by the HCRTR1 gene.

Orexin receptor type 2 (Ox2R or OX2), also known as hypocretin receptor type 2 (HcrtR2), is a protein that in humans is encoded by the HCRTR2 gene.

Almorexant, also known by its development code ACT-078573, is an orexin antagonist, acting as a competitive antagonist of the OX1 and OX2 orexin receptors, which was being developed by the pharmaceutical companies Actelion and GSK for the treatment of insomnia. Development of the drug was abandoned in January 2011 due to concerns over the hepatic safety of almorexant after transient increases in liver enzymes were observed in trials.
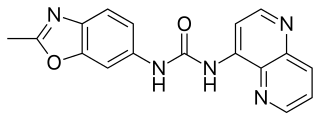
SB-334867 is an orexin antagonist. It was the first non-peptide antagonist developed that is selective for the orexin receptor subtype OX1, with around 50x selectivity for OX1 over OX2 receptors. It has been shown to produce sedative and anorectic effects in animals, and has been useful in characterising the orexinergic regulation of brain systems involved with appetite and sleep, as well as other physiological processes. The hydrochloride salt of SB-334867 has been demonstrated to be hydrolytically unstable, both in solution and as the solid. Orexin antagonists have multiple potential clinical applications including the treatment of drug addiction, insomnia, obesity and diabetes.

Suvorexant, sold under the brand name Belsomra, is an orexin antagonist medication which is used in the treatment of insomnia. It is indicated specifically for the treatment of insomnia characterized by difficulties with sleep onset and/or maintenance in adults. Suvorexant helps with falling asleep faster, sleeping longer, being awake less in the middle of the night, and having better quality of sleep. Its effectiveness is modest, and is similar to that of other orexin antagonists, but is lower than that of benzodiazepines and Z-drugs. Suvorexant is taken by mouth.
An orexin receptor antagonist, or orexin antagonist, is a drug that inhibits the effect of orexin by acting as a receptor antagonist of one (selective orexin receptor antagonist or SORA) or both (dual orexin receptor antagonis or DORA) of the orexin receptors, OX1 and OX2. Medical applications include treatment of sleep disorders such as insomnia.

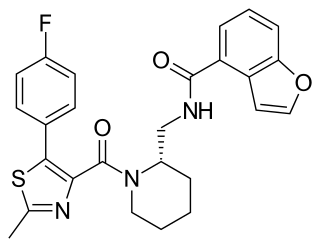
Aticaprant, also known by its developmental codes JNJ-67953964, CERC-501, and LY-2456302, is a κ-opioid receptor (KOR) antagonist which is under development for the treatment of major depressive disorder. A regulatory application for approval of the medication is expected to be submitted by 2025. Aticaprant is taken by mouth.

Filorexant (INN, USAN; developmental code name MK-6096) is an orexin antagonist which was under development by Merck for the treatment of insomnia, depression, diabetic neuropathy, and migraine. It is a dual antagonist of the orexin OX1 and OX2 receptors. It has a relatively short elimination half-life of 3 to 6 hours. However, it dissociates slowly from the orexin receptors and may thereby have a longer duration. Possibly in relation to this, filorexant shows next-day somnolence similarly to suvorexant. In phase 2 clinical trials, filorexant was found to be effective in the treatment of insomnia, but was not effective in the treatment of major depressive disorder, painful diabetic neuropathy, or migraine. As of May 2015, filorexant was no longer listed on Merck's online development pipeline and hence development of the drug appears to have been discontinued. Development of filorexant may have been discontinued due to lack of differentiation from suvorexant (which was also developed by Merck).

BTRX-246040, also known as LY-2940094, is a potent and selective nociceptin receptor antagonist which is under development by BlackThorn Therapeutics and Eli Lilly for the treatment of major depressive disorder (MDD). It has demonstrated proof-of-concept clinical efficacy for depression. As of 2017, it is in phase II clinical trials for the treatment of MDD. It was also under investigation for the treatment of alcoholism, and similarly reached phase II clinical studies for this indication, but development was discontinued.

MIN-117 is an investigational antidepressant which is under development by Minerva Neurosciences for the clinical treatment of major depressive disorder (MDD). It is described as a 5-HT1A and 5-HT2A receptor antagonist and inhibitor of serotonin and dopamine reuptake, and is also reported to possess affinity for the α1A- and α1B-adrenergic receptors. As of May 2015, MIN-117 is in phase II clinical trials for MDD. In December 2019, Minerva announced that MIN-117 was no longer in clinical development for MDD after disappointing results in a phase IIb trial.

Lemborexant, sold under the brand name Dayvigo, is an orexin antagonist medication which is used in the treatment of insomnia. It is indicated specifically for the treatment of insomnia characterized by difficulties with sleep onset and/or maintenance in adults. The medication is taken by mouth.

Daridorexant, sold under the brand name Quviviq, is an orexin antagonist medication which is used for the treatment of insomnia. Daridorexant is taken by mouth.

Vornorexant, also known by its developmental code names ORN-0829 and TS-142, is an orexin antagonist medication which is under development for the treatment of insomnia and sleep apnea. It is a dual orexin OX1 and OX2 receptor antagonist (DORA). The medication is taken by mouth. As of June 2021, vornorexant is in phase 2 clinical trials for insomnia and phase 1 trials for sleep apnea. It is under development by Taisho Pharmaceutical.

Firazorexton (INN; development code TAK-994) is an experimental orexin 2 (OX2) receptor agonist first described in a 2019 patent filed by Takeda Pharmaceutical Company.

ACT-539313 is an orexin antagonist medication which is under development for the treatment of binge eating disorder and was previously under development for the treatment of anxiety disorders. It is an orally active small-molecule compound with an elimination half-life of 3.3 to 6.5 hours and acts as a selective orexin OX1 receptor antagonist (1-SORA). As of May 2022, the drug is in phase 2 clinical trials for binge eating disorder. Following negative efficacy results of a phase 2 trial of ACT-539313 for binge eating disorder, Idorsia (the developer of ACT-539313) signaled in May 2022 that it would not pursue further development of the drug for this indication.

JNJ-61393215 is an orexin antagonist medication which is under development for the treatment of depression and anxiety disorders. It is an orally active compound and acts as a selective antagonist of the orexin OX1 receptor (1-SORA). Preliminary clinical findings suggest that JNJ-61393215 may have anti-panic effects in humans. As of November 2021, JNJ-61393215 is in phase 2 clinical trials for the treatment of major depressive disorder and is in the preclinical stage of development for treatment of panic disorder, while no further development has been reported for treatment of other anxiety disorders. The drug was originated and developed by Janssen Pharmaceuticals.