Orexin, also known as hypocretin, is a neuropeptide that regulates arousal, wakefulness, and appetite. The most common form of narcolepsy, type 1, in which the individual experiences brief losses of muscle tone, is caused by a lack of orexin in the brain due to destruction of the cells that produce it. It exists in the forms of orexin-A and orexin-B.
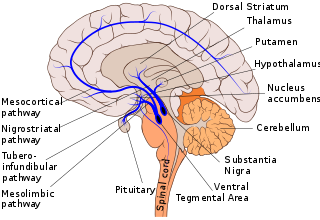
Dopaminergic pathways in the human brain are involved in both physiological and behavioral processes including movement, cognition, executive functions, reward, motivation, and neuroendocrine control. Each pathway is a set of projection neurons, consisting of individual dopaminergic neurons.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are endogenous lipid-based retrograde neurotransmitters that bind to cannabinoid receptors, and cannabinoid receptor proteins that are expressed throughout the vertebrate central nervous system and peripheral nervous system. The endocannabinoid system remains under preliminary research, but may be involved in regulating physiological and cognitive processes, including fertility, pregnancy, pre- and postnatal development, various activity of immune system, appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.

The lateral hypothalamus (LH), also called the lateral hypothalamic area (LHA), contains the primary orexinergic nucleus within the hypothalamus that widely projects throughout the nervous system; this system of neurons mediates an array of cognitive and physical processes, such as promoting feeding behavior and arousal, reducing pain perception, and regulating body temperature, digestive functions, and blood pressure, among many others. Clinically significant disorders that involve dysfunctions of the orexinergic projection system include narcolepsy, motility disorders or functional gastrointestinal disorders involving visceral hypersensitivity, and eating disorders.
The orexin receptor (also referred to as the hypocretin receptor) is a G-protein-coupled receptor that binds the neuropeptide orexin. There are two variants, OX1 and OX2, each encoded by a different gene (HCRTR1, HCRTR2).

SB-277,011A is a drug which acts as a potent and selective dopamine D3 receptor antagonist, which is around 80-100x selective for D3 over D2, and lacks any partial agonist activity.

Orexin receptor type 1 (Ox1R or OX1), also known as hypocretin receptor type 1 (HcrtR1), is a protein that in humans is encoded by the HCRTR1 gene.

Orexin receptor type 2 (Ox2R or OX2), also known as hypocretin receptor type 2 (HcrtR2), is a protein that in humans is encoded by the HCRTR2 gene.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.
Orexin-A, also known as hypocretin-1, is a naturally occurring neuropeptide and orexin isoform. The orexinergic nucleus in the lateral hypothalamus is the primary orexin projection system in the brain.

Almorexant, also known by its development code ACT-078573, is an orexin antagonist, acting as a competitive antagonist of the OX1 and OX2 orexin receptors, which was being developed by the pharmaceutical companies Actelion and GSK for the treatment of insomnia. Development of the drug was abandoned in January 2011 due to concerns over the hepatic safety of almorexant after transient increases in liver enzymes were observed in trials.

SB-271046 is a drug which is used in scientific research. It was one of the first selective 5-HT6 receptor antagonists to be discovered, and was found through high-throughput screening of the SmithKline Beecham Compound Bank using cloned 5-HT6 receptors as a target, with an initial lead compound being developed into SB-271046 through a structure-activity relationship (SAR) study. SB-271046 was found to be potent and selective in vitro and had good oral bioavailability in vivo, but had poor penetration across the blood–brain barrier, so further SAR work was then conducted, which led to improved 5-HT6 antagonists such as SB-357,134 and SB-399,885.

SB-216641 is a drug which is a selective antagonist for the serotonin receptor 5-HT1B, with around 25x selectivity over the closely related 5-HT1D receptor. It is used in scientific research, and has demonstrated anxiolytic effects in animal studies.
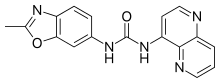
SB-408124 is a drug which is a non-peptide antagonist selective for the orexin receptor subtype OX1, with around 70x selectivity for OX1 over OX2 receptors, and improved oral bioavailability compared to the older OX1 antagonist SB-334867. It is used in scientific research into the function of orexinergic neurons in the body.

Suvorexant, sold under the brand name Belsomra, is an orexin antagonist medication which is used in the treatment of insomnia. It is indicated specifically for the treatment of insomnia characterized by difficulties with sleep onset and/or maintenance in adults. Suvorexant helps with falling asleep faster, sleeping longer, being awake less in the middle of the night, and having better quality of sleep. Its effectiveness is modest, and is similar to that of other orexin antagonists, but is lower than that of benzodiazepines and Z-drugs. Suvorexant is taken by mouth.
An orexin receptor antagonist, or orexin antagonist, is a drug that inhibits the effect of orexin by acting as a receptor antagonist of one (selective orexin receptor antagonist or SORA) or both (dual orexin receptor antagonis or DORA) of the orexin receptors, OX1 and OX2. Medical applications include treatment of sleep disorders such as insomnia.

Filorexant (INNTooltip International Nonproprietary Name, USANTooltip United States Approved Name; developmental code name MK-6096) is an orexin antagonist which was under development by Merck for the treatment of insomnia, depression, diabetic neuropathy, and migraine. It is a dual antagonist of the orexin OX1 and OX2 receptors. It has a relatively short elimination half-life of 3 to 6 hours. However, it dissociates slowly from the orexin receptors and may thereby have a longer duration. Possibly in relation to this, filorexant shows next-day somnolence similarly to suvorexant. In phase 2 clinical trials, filorexant was found to be effective in the treatment of insomnia, but was not effective in the treatment of major depressive disorder, painful diabetic neuropathy, or migraine. As of May 2015, filorexant was no longer listed on Merck's online development pipeline and hence development of the drug appears to have been discontinued. Development of filorexant may have been discontinued due to lack of differentiation from suvorexant (which was also developed by Merck).

Seltorexant, also known by its developmental code names MIN-202 and JNJ-42847922, is an orexin antagonist medication which is under development for the treatment of depression and insomnia. It is a selective antagonist of the orexin OX2 receptor (2-SORA). The medication is taken by mouth. As of February 2022, seltorexant is in phase 3 clinical trials for treatment of major depressive disorder (MDD) and phase 2 trials for treatment of insomnia. It was also under investigation for the treatment of sleep apnea, but no recent development has been reported for this indication. Seltorexant is under development by Minerva Neurosciences and Johnson & Johnson's Janssen Pharmaceuticals.

ACT-539313 is an orexin antagonist medication which is under development for the treatment of binge eating disorder and was previously under development for the treatment of anxiety disorders. It is an orally active small-molecule compound with an elimination half-life of 3.3 to 6.5 hours and acts as a selective orexin OX1 receptor antagonist (1-SORA). As of May 2022, the drug is in phase 2 clinical trials for binge eating disorder. Following negative efficacy results of a phase 2 trial of ACT-539313 for binge eating disorder, Idorsia (the developer of ACT-539313) signaled in May 2022 that it would not pursue further development of the drug for this indication.