Meconium aspiration syndrome (MAS), also known as neonatal aspiration of meconium, is a medical condition affecting newborn infants. It describes the spectrum of disorders and pathophysiology of newborns born in meconium-stained amniotic fluid (MSAF) and have meconium within their lungs. Therefore, MAS has a wide range of severity depending on what conditions and complications develop after parturition. Furthermore, the pathophysiology of MAS is multifactorial and extremely complex which is why it is the leading cause of morbidity and mortality in term infants.

The lungs are the primary organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the backbone on either side of the heart. Their function in the respiratory system is to extract oxygen from the atmosphere and transfer it into the bloodstream, and to release carbon dioxide from the bloodstream into the atmosphere, in a process of gas exchange. Respiration is driven by different muscular systems in different species. Mammals, reptiles and birds use their musculoskeletal systems to support and foster breathing. In early tetrapods, air was driven into the lungs by the pharyngeal muscles via buccal pumping, a mechanism still seen in amphibians. In humans, the primary muscle that drives breathing is the diaphragm. The lungs also provide airflow that makes vocalisation including speech possible.

The respiratory system is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies greatly, depending on the size of the organism, the environment in which it lives and its evolutionary history. In land animals, the respiratory surface is internalized as linings of the lungs. Gas exchange in the lungs occurs in millions of small air sacs; in mammals and reptiles, these are called alveoli, and in birds, they are known as atria. These microscopic air sacs have a very rich blood supply, thus bringing the air into close contact with the blood. These air sacs communicate with the external environment via a system of airways, or hollow tubes, of which the largest is the trachea, which branches in the middle of the chest into the two main bronchi. These enter the lungs where they branch into progressively narrower secondary and tertiary bronchi that branch into numerous smaller tubes, the bronchioles. In birds, the bronchioles are termed parabronchi. It is the bronchioles, or parabronchi that generally open into the microscopic alveoli in mammals and atria in birds. Air has to be pumped from the environment into the alveoli or atria by the process of breathing which involves the muscles of respiration.

A pulmonary alveolus, also called an air sac or air space, is one of millions of hollow, distensible cup-shaped cavities in the lungs where pulmonary gas exchange takes place. Oxygen is exchanged for carbon dioxide at the blood–air barrier between the alveolar air and the pulmonary capillary. Alveoli make up the functional tissue of the mammalian lungs known as the lung parenchyma, which takes up 90 percent of the total lung volume.

Infant respiratory distress syndrome (IRDS), also known as surfactant deficiency disorder (SDD), and previously called hyaline membrane disease (HMD), is a syndrome in premature infants caused by developmental insufficiency of pulmonary surfactant production and structural immaturity in the lungs. It can also be a consequence of neonatal infection and can result from a genetic problem with the production of surfactant-associated proteins.

Pulmonary surfactant is a surface-active complex of phospholipids and proteins formed by type II alveolar cells. The proteins and lipids that make up the surfactant have both hydrophilic and hydrophobic regions. By adsorbing to the air-water interface of alveoli, with hydrophilic head groups in the water and the hydrophobic tails facing towards the air, the main lipid component of surfactant, dipalmitoylphosphatidylcholine (DPPC), reduces surface tension.
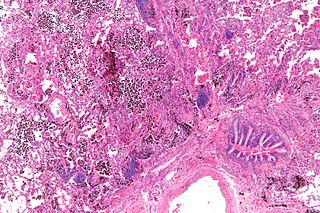
Pulmonary hemorrhage is an acute bleeding from the lung, from the upper respiratory tract and the trachea, and the pulmonary alveoli. When evident clinically, the condition is usually massive. The onset of pulmonary hemorrhage is characterized by a cough productive of blood (hemoptysis) and worsening of oxygenation leading to cyanosis. Treatment should be immediate and should include tracheal suction, oxygen, positive pressure ventilation, and correction of underlying abnormalities such as disorders of coagulation. A blood transfusion may be necessary.
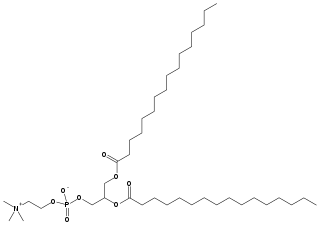
Dipalmitoylphosphatidylcholine (DPPC) is a phospholipid (and a lecithin) consisting of two C16 palmitic acid groups attached to a phosphatidylcholine head-group.

In cell biology, lamellar bodies are secretory organelles found in type II alveolar cells in the lungs, and in keratinocytes in the skin. They are oblong structures, appearing about 300-400 nm in width and 100-150 nm in length in transmission electron microscopy images. Lamellar bodies in the alveoli of the lungs fuse with the cell membrane and release pulmonary surfactant into the extracellular space.

Surfactant protein D, also known as SP-D, is a lung surfactant protein part of the collagenous family of lectins called collectin. In humans, SP-D is encoded by the SFTPD gene and is part of the innate immune system. Each SP-D subunit is composed of an N-terminal domain, a collagenous region, a nucleating neck region, and a C-terminal lectin domain. Three of these subunits assemble to form a homotrimer, which further assemble into a tetrameric complex.

Surfactant protein C (SP-C), is one of the pulmonary surfactant proteins. In humans this is encoded by the SFTPC gene.

Surfactant protein A1(SP-A1), also known as Pulmonary surfactant-associated protein A1(PSP-A) is a protein that in humans is encoded by the SFTPA1 gene.

Surfactant protein A2(SP-A2), also known as Pulmonary surfactant-associated protein A2(PSP-A2) is a protein that in humans is encoded by the SFTPA2 gene.

The lecithin–sphingomyelin ratio is a test of fetal amniotic fluid to assess for fetal lung immaturity. Lungs require surfactant, a soap-like substance, to lower the surface tension of the fluid coating the alveolar epithelium in the lungs. This is especially important for premature babies trying to expand their lungs after birth. Surfactant is a mixture of lipids, proteins, and glycoproteins, lecithin and sphingomyelin being two of them. Lecithin makes the surfactant mixture more effective.

Diffuse alveolar damage (DAD) is a histologic term used to describe specific changes that occur to the structure of the lungs during injury or disease. Most often DAD is described in association with the early stages of acute respiratory distress syndrome (ARDS). DAD can be seen in situations other than ARDS and that ARDS can occur without DAD.
Poractant alfa is a pulmonary surfactant sold under the brand name Curosurf by Chiesi Farmaceutici. Poractant alfa is an extract of natural porcine lung surfactant. As with other surfactants, marked improvement on oxygenation may occur within minutes of the administration of poractant alfa. The new generic form of surfactant is Varasurf developed in PersisGen Co. and commercialized by ArnaGen Pharmad. It has fully comparable quality profile with Curosurf.
Surfactant metabolism dysfunction is a condition where pulmonary surfactant is insufficient for adequate respiration. Surface tension at the liquid-air interphase in the alveoli makes the air sacs prone to collapsing post expiration. This is due to the fact that water molecules in the liquid-air surface of alveoli are more attracted to one another than they are to molecules in the air. For sphere-like structures like alveoli, water molecules line the inner walls of the air sacs and stick tightly together through hydrogen bonds. These intermolecular forces put great restraint on the inner walls of the air sac, tighten the surface all together, and unyielding to stretch for inhalation. Thus, without something to alleviate this surface tension, alveoli can collapse and cannot be filled up again. Surfactant is essential mixture that is released into the air-facing surface of inner walls of air sacs to lessen the strength of surface tension. This mixture inserts itself among water molecules and breaks up hydrogen bonds that hold the tension. Multiple lung diseases, like ISD or RDS, in newborns and late-onsets cases have been linked to dysfunction of surfactant metabolism.

Pulmonary surfactant is used as a medication to treat and prevent respiratory distress syndrome in newborn babies.
Whole lung lavage (WLL), also called lung washing, is a medical procedure in which the patient's lungs are washed with saline by filling and draining repeatedly. It is used to treat pulmonary alveolar proteinosis, in which excess lung surfactant proteins prevent the patient from breathing. Some sources consider it a variation of bronchoalveolar lavage.
Development of the respiratory system begins early in the fetus. It is a complex process that includes many structures, most of which arise from the endoderm. Towards the end of development, the fetus can be observed making breathing movements. Until birth, however, the mother provides all of the oxygen to the fetus as well as removes all of the fetal carbon dioxide via the placenta.