Thallium(III) hydroxide, Tl(OH)3, also known as thallic hydroxide, is a hydroxide of thallium. It is a white solid.

Mercury(II) sulfate, commonly called mercuric sulfate, is the chemical compound HgSO4. It is an odorless salt that forms white granules or crystalline powder. In water, it separates into an insoluble basic sulfate with a yellow color and sulfuric acid.
Indium(III) sulfate (In2(SO4)3) is a sulfate salt of the metal indium. It is a sesquisulfate, meaning that the sulfate group occurs 11/2 times as much as the metal. It may be formed by the reaction of indium, its oxide, or its carbonate with sulfuric acid. An excess of strong acid is required, otherwise insoluble basic salts are formed. As a solid indium sulfate can be anhydrous, or take the form of a pentahydrate with five water molecules or a nonahydrate with nine molecules of water. Indium sulfate is used in the production of indium or indium containing substances. Indium sulfate also can be found in basic salts, acidic salts or double salts including indium alum.
Tutton's salts are a family of salts with the formula M2M'(SO4)2(H2O)6 (sulfates) or M2M'(SeO4)2(H2O)6 (selenates). These materials are double salts, which means that they contain two different cations, M+ and M'2+ crystallized in the same regular ionic lattice. The univalent cation can be potassium, rubidium, caesium, ammonium (NH4), deuterated ammonium (ND4) or thallium. Sodium or lithium ions are too small. The divalent cation can be magnesium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc or cadmium. In addition to sulfate and selenate, the divalent anion can be chromate (CrO42−), tetrafluoroberyllate (BeF42−), hydrogenphosphate (HPO42−) or monofluorophosphate (PO3F2−). Tutton's salts crystallize in the monoclinic space group P21/a. The robustness is the result of the complementary hydrogen-bonding between the tetrahedral anions and cations as well their interactions with the metal aquo complex [M(H2O)6]2+.
Langbeinites are a family of crystalline substances based on the structure of langbeinite with general formula M2M'2(SO4)3, where M is a large univalent cation, and M' is a small divalent cation. The sulfate group, SO2−4, can be substituted by other tetrahedral anions with a double negative charge such as tetrafluoroberyllate, selenate, chromate, molybdate, or tungstates. Although monofluorophosphates are predicted, they have not been described. By redistributing charges other anions with the same shape such as phosphate also form langbeinite structures. In these the M' atom must have a greater charge to balance the extra three negative charges.

Scandium triiodide, also known as scandium iodide, is an inorganic compound with the formula ScI3 and is classified as a lanthanide iodide. This salt is a yellowish powder. It is used in metal halide lamps together with similar compounds, such as caesium iodide, because of their ability to maximize emission of UV and to prolong bulb life. The maximized UV emission can be tuned to a range that can initiate photopolymerizations.
Zirconium perchlorate is a molecular substance containing zirconium and perchlorate groups with formula Zr(ClO4)4. Zr(ClO4)4 is a volatile crystalline product. It can be formed by reacting zirconium tetrachloride with dry perchloric acid at liquid nitrogen temperatures. Zr(ClO4)4 sublimes slowly in a vacuum at 70°C showing that the molecule is covalently bound rather than being ionic. The reaction also forms some zirconyl perchlorate (or zirconium oxyperchlorate) ZrO(ClO4)2 as even apparently pure perchloric acid is in equilibrium with dichlorine heptoxide, hydronium ions and perchlorate ions. This side product can be minimised by adding more dichlorine heptoxide or doing the reaction as cold as possible.

Neodymium(III) bromide is an inorganic salt of bromine and neodymium the formula NdBr3. The anhydrous compound is an off-white to pale green solid at room temperature, with an orthorhombic PuBr3-type crystal structure. The material is hygroscopic and forms a hexahydrate in water (NdBr3· 6H2O), similar to the related neodymium(III) chloride.
Iron(II) selenate (ferrous selenate) is an inorganic compound with the formula FeSeO4. It has anhydrous and several hydrate forms. The pentahydrate has the structure, [Fe(H2O)4]SeO4•H2O, isomorphous to the corresponding iron(II) sulfate. Heptahydrate is also known, in form of unstable green crystalline solid.
The sulfate fluorides are double salts that contain both sulfate and fluoride anions. They are in the class of mixed anion compounds. Some of these minerals are deposited in fumaroles.
Scandium(III) hydroxide is an inorganic compound with the chemical formula Sc(OH)3, the trivalent hydroxide of scandium. It is an amphoteric compound. It is slightly soluble in water, and its saturated solution contains Sc(OH)3 and a small amount of Sc(OH)+2. The solubility of scandium(III) hydroxide in water is 0.0279 mol/L. It will convert to ScO(OH) after aging, greatly reducing the solubility (0.0008 mol/L). Scandium(III) hydroxide can be produced by reacting scandium salts and alkali hydroxides. In the reaction, different starting ingredients can generate different intermediates such as Sc(OH)1.75Cl1.25, Sc(OH)2NO3 and Sc(OH)2.32(SO4)0.34.
Gallium(III) sulfate refers to the chemical compound, a salt, with the formula Ga2(SO4)3, or its hydrates Ga2(SO4)3·xH2O. Gallium metal dissolves in sulfuric acid to form solutions containing [Ga(OH2)6]3+ and SO42− ions. The octadecahydrate Ga2(SO4)3·18H2O crystallises from these solutions at room temperature. This hydrate loses water in stages when heated, forming the anhydrate Ga2(SO4)3 above 150 °C and completely above 310 °C. Anhydrous Ga2(SO4)3 is isostructural with iron(III) sulfate, crystallizing in the rhombohedral space group R3.
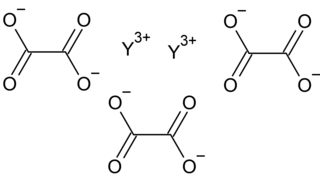
Yttrium oxalate is an inorganic compound, a salt of yttrium and oxalic acid with the chemical formula Y2(C2O4)3. The compound does not dissolve in water and forms crystalline hydrates—colorless crystals.

Neodymium perrhenate is an inorganic compound with the chemical formula Nd(ReO4)3, which exists in anhydrous and tetrahydrate. It can be obtained by reacting excess neodymium oxide with 240 g/L perrhenic acid solution. In its solution, NdReO42+ and Nd(ReO4)2+ can be observed with stability constants of 16.5 and 23.6, respectively.

Neodymium(III) acetylacetonate is a coordination compound with the chemical formula Nd(O2C5H7)3. Although many sources discuss this anhydrous acetylacetonate complex, it is the dihydrate Nd(O2C5H7)3(H2O)2 that has been characterized by X-ray crystallography. It commonly occurs as a white powder. Upon heating under vacuum, other dihydrated lanthanide trisacetylacetonates convert to oxo-clusters M4O(C5H7O2)10. This result suggests that Nd(O2C5H7)3 may not exist.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Praseodymium acetylacetonate is a coordination complex with the formula Pr(C3H7O2)3. This purported anhydrous acetylacetonate complex is widely discussed but only the dihydrate Pr(C3H7O2)3(H2O)2 has been characterized by X-ray crystallography.
Praseodymium bromide is an inorganic compound with the chemical formula Pr(BrO3)3. It is soluble in water and can form the dihydrate, tetrahydrate and nonahydrate. The nonahydrate melts in its own crystal water at 56.5 °C and completely loses its crystal water at 130 °C. It can be produced by the reaction of barium bromate and praseodymium sulfate.
Thulium(III) telluride is an inorganic compound, one of the tellurides of thulium, with the chemical formula Tm2Te3. It is an orthorhombic crystal with space group Fddd. It can dissolve in lead telluride at high temperatures to form a solid solution.








