Interferons are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.

The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases.
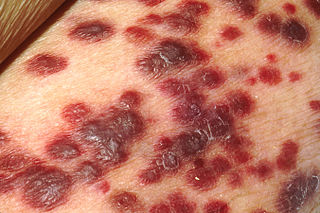
Kaposi's sarcoma-associated herpesvirus (KSHV) is the ninth known human herpesvirus; its formal name according to the International Committee on Taxonomy of Viruses (ICTV) is Human gammaherpesvirus 8, or HHV-8 in short. Like other herpesviruses, its informal names are used interchangeably with its formal ICTV name. This virus causes Kaposi's sarcoma, a cancer commonly occurring in AIDS patients, as well as primary effusion lymphoma, HHV-8-associated multicentric Castleman's disease and KSHV inflammatory cytokine syndrome. It is one of seven currently known human cancer viruses, or oncoviruses. Even after many years since the discovery of KSHV/HHV8, there is no known cure for KSHV associated tumorigenesis.
Primary effusion lymphoma (PEL) is classified as a diffuse large B cell lymphoma. It is a rare malignancy of plasmablastic cells that occurs in individuals that are infected with the Kaposi's sarcoma-associated herpesvirus. Plasmablasts are immature plasma cells, i.e. lymphocytes of the B-cell type that have differentiated into plasmablasts but because of their malignant nature do not differentiate into mature plasma cells but rather proliferate excessively and thereby cause life-threatening disease. In PEL, the proliferating plasmablastoid cells commonly accumulate within body cavities to produce effusions, primarily in the pleural, pericardial, or peritoneal cavities, without forming a contiguous tumor mass. In rare cases of these cavitary forms of PEL, the effusions develop in joints, the epidural space surrounding the brain and spinal cord, and underneath the capsule which forms around breast implants. Less frequently, individuals present with extracavitary primary effusion lymphomas, i.e., solid tumor masses not accompanied by effusions. The extracavitary tumors may develop in lymph nodes, bone, bone marrow, the gastrointestinal tract, skin, spleen, liver, lungs, central nervous system, testes, paranasal sinuses, muscle, and, rarely, inside the vasculature and sinuses of lymph nodes. As their disease progresses, however, individuals with the classical effusion-form of PEL may develop extracavitary tumors and individuals with extracavitary PEL may develop cavitary effusions.
The type III interferon group is a group of anti-viral cytokines, that consists of four IFN-λ (lambda) molecules called IFN-λ1, IFN-λ2, IFN-λ3, and IFN-λ4. They were discovered in 2003. Their function is similar to that of type I interferons, but is less intense and serves mostly as a first-line defense against viruses in the epithelium.

Signal transducer and activator of transcription 1 (STAT1) is a transcription factor which in humans is encoded by the STAT1 gene. It is a member of the STAT protein family.

Interferon regulatory factor 3, also known as IRF3, is an interferon regulatory factor.
NSP1 (NS53), the product of rotavirus gene 5, is a nonstructural RNA-binding protein that contains a cysteine-rich region and is a component of early replication intermediates. RNA-folding predictions suggest that this region of the NSP1 mRNA can interact with itself, producing a stem-loop structure similar to that found near the 5'-terminus of the NSP1 mRNA.

Interferon regulatory factor 2 is a protein that in humans is encoded by the IRF2 gene.

RIG-I is a cytosolic pattern recognition receptor (PRR) that can mediate induction of a type-I interferon (IFN1) response. RIG-I is an essential molecule in the innate immune system for recognizing cells that have been infected with a virus. These viruses can include West Nile virus, Japanese Encephalitis virus, influenza A, Sendai virus, flavivirus, and coronaviruses.
Interferon regulatory factor 1 is a protein that in humans is encoded by the IRF1 gene.

Interferon alpha-1 is a protein that in humans is encoded by the IFNA1 gene.

Interferon alpha-2 is a protein that in humans is encoded by the IFNA2 gene.

Interferon regulatory factor 4 (IRF4) also known as MUM1 is a protein that in humans is encoded by the IRF4 gene. IRF4 functions as a key regulatory transcription factor in the development of human immune cells. The expression of IRF4 is essential for the differentiation of T lymphocytes and B lymphocytes as well as certain myeloid cells. Dysregulation of the IRF4 gene can result in IRF4 functioning either as an oncogene or a tumor-suppressor, depending on the context of the modification.

Interferon regulatory factor 5 is a protein that in humans is encoded by the IRF5 gene. The IRF family is a group of transcription factors that are involved in signaling for virus responses in mammals along with regulation of certain cellular functions.

PR domain zinc finger protein 1, or B lymphocyte-induced maturation protein-1 (BLIMP-1), is a protein in humans encoded by the gene PRDM1 located on chromosome 6q21. BLIMP-1 is considered a 'master regulator' of hematopoietic stem cells, and plays a critical role in the development of plasma B cells, T cells, dendritic cells (DCs), macrophages, and osteoclasts. Pattern Recognition Receptors (PRRs) can activate BLIMP-1, both as a direct target and through downstream activation. BLIMP-1 is a transcription factor that triggers expression of many downstream signaling cascades. As a fine-tuned and contextual rheostat of the immune system, BLIMP-1 up- or down-regulates immune responses depending on the precise scenarios. BLIMP-1 is highly expressed in exhausted T-cells – clones of dysfunctional T-cells with diminished functions due to chronic immune response against cancer, viral infections, or organ transplant.

Interferon regulatory factor 8 (IRF8) also known as interferon consensus sequence-binding protein (ICSBP), is a protein that in humans is encoded by the IRF8 gene. IRF8 is a transcription factor that plays critical roles in the regulation of lineage commitment and in myeloid cell maturation including the decision for a common myeloid progenitor (CMP) to differentiate into a monocyte precursor cell.

OX-2 membrane glycoprotein, also named CD200 is a human protein encoded by the CD200 gene. CD200 gene is in human located on chromosome 3 in proximity to genes encoding other B7 proteins CD80/CD86. In mice CD200 gene is on chromosome 16.

Kaposi's sarcoma (KS) is a type of cancer that can form masses in the skin, in lymph nodes, in the mouth, or in other organs. The skin lesions are usually painless, purple and may be flat or raised. Lesions can occur singly, multiply in a limited area, or may be widespread. Depending on the sub-type of disease and level of immune suppression, KS may worsen either gradually or quickly. Except for Classical KS where there is generally no immune suppression, KS is caused by a combination of immune suppression and infection by Human herpesvirus 8.
Type 1 regulatory cells or Tr1 (TR1) cells are a class of regulatory T cells participating in peripheral immunity as a subsets of CD4+ T cells. Tr1 cells regulate tolerance towards antigens of any origin. Tr1 cells are self or non-self antigen specific and their key role is to induce and maintain peripheral tolerance and suppress tissue inflammation in autoimmunity and graft vs. host disease.