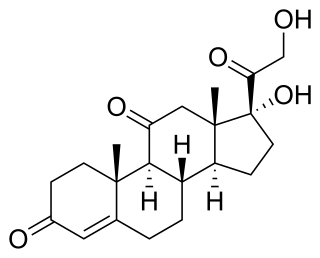
Cortisone is a pregnene (21-carbon) steroid hormone. It is a naturally-occurring corticosteroid metabolite that is also used as a pharmaceutical prodrug. Cortisol is converted by the action of the enzyme corticosteroid 11-beta-dehydrogenase isozyme 2 into the inactive metabolite cortisone, particularly in the kidneys. This is done by oxidizing the alcohol group at carbon 11. Cortisone is converted back to the active steroid cortisol by stereospecific hydrogenation at carbon 11 by the enzyme 11β-Hydroxysteroid dehydrogenase type 1, particularly in the liver.

Cortisol is a steroid hormone, in the glucocorticoid class of hormones and a stress hormone. When used as a medication, it is known as hydrocortisone.

Hydrocortisone is the name for the hormone cortisol when supplied as a medication. Uses include conditions such as adrenocortical insufficiency, adrenogenital syndrome, high blood calcium, thyroiditis, rheumatoid arthritis, dermatitis, asthma, and COPD. It is the treatment of choice for adrenocortical insufficiency. It can be given by mouth, topically, or by injection. Stopping treatment after long-term use should be done slowly.

Estriol (E3), also spelled oestriol, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estrone. Levels of estriol in women who are not pregnant are almost undetectable. However, during pregnancy, estriol is synthesized in very high quantities by the placenta and is the most produced estrogen in the body by far, although circulating levels of estriol are similar to those of other estrogens due to a relatively high rate of metabolism and excretion. Relative to estradiol, both estriol and estrone have far weaker activity as estrogens.

Cytochromes P450 are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Estabrook, Cooper, and Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids, and hormones.

Cytochrome P450 3A4 is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by CYP3A4 gene. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme.

Triamcinolone is a glucocorticoid used to treat certain skin diseases, allergies, and rheumatic disorders among others. It is also used to prevent worsening of asthma and COPD. It can be taken in various ways including by mouth, injection into a muscle, and inhalation.

Enoxolone is a pentacyclic triterpenoid derivative of the beta-amyrin type obtained from the hydrolysis of glycyrrhizic acid, which was obtained from the herb liquorice.
11β-Hydroxysteroid dehydrogenase enzymes catalyze the conversion of inert 11 keto-products (cortisone) to active cortisol, or vice versa, thus regulating the access of glucocorticoids to the steroid receptors.
Cytochrome P450 1A2, a member of the cytochrome P450 mixed-function oxidase system, is involved in the metabolism of xenobiotics in the human body. In humans, the CYP1A2 enzyme is encoded by the CYP1A2 gene.

11β-Hydroxysteroid dehydrogenase type 1, also known as cortisone reductase, is an NADPH-dependent enzyme highly expressed in key metabolic tissues including liver, adipose tissue, and the central nervous system. In these tissues, HSD11B1 reduces cortisone to the active hormone cortisol that activates glucocorticoid receptors. It belongs to the family of short-chain dehydrogenases. It is encoded by the HSD11B1 gene.
3β-Hydroxysteroid dehydrogenase/Δ5-4 isomerase (3β-HSD) is an enzyme that catalyzes the biosynthesis of the steroid progesterone from pregnenolone, 17α-hydroxyprogesterone from 17α-hydroxypregnenolone, and androstenedione from dehydroepiandrosterone (DHEA) in the adrenal gland. It is the only enzyme in the adrenal pathway of corticosteroid synthesis that is not a member of the cytochrome P450 family. It is also present in other steroid-producing tissues, including the ovary, testis and placenta. In humans, there are two 3β-HSD isozymes encoded by the HSD3B1 and HSD3B2 genes.
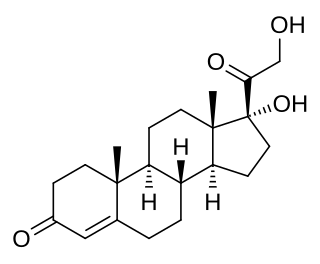
11-Deoxycortisol, also known as cortodoxone (INN), cortexolone as well as 17α,21-dihydroxyprogesterone or 17α,21-dihydroxypregn-4-ene-3,20-dione, is an endogenous glucocorticoid steroid hormone, and a metabolic intermediate toward cortisol. It was first described by Tadeusz Reichstein in 1938 as Substance S, thus has also been referred to as Reichstein's Substance S or Compound S.

Cytochrome P450 3A5 is a protein that in humans is encoded by the CYP3A5 gene.

5α-Dihydroprogesterone is an endogenous progestogen and neurosteroid that is synthesized from progesterone. It is also an intermediate in the synthesis of allopregnanolone and isopregnanolone from progesterone.

3-Methoxymorphinan is a levomethorphan metabolite that has been shown to produce local anesthetic effects. It is the CYP3A4 metabolite of dextromethorphan, and is itself metabolized by the liver enzyme CYP2D6.
Cortisone reductase deficiency is caused by dysregulation of the 11β-hydroxysteroid dehydrogenase type 1 enzyme (11β-HSD1), otherwise known as cortisone reductase, a bi-directional enzyme, which catalyzes the interconversion of cortisone to cortisol in the presence of NADH as a co-factor. If levels of NADH are low, the enzyme catalyses the reverse reaction, from cortisol to cortisone, using NAD+ as a co-factor.
Cortisol is a glucocorticoid that plays a variety of roles in many different biochemical pathways, including, but not limited to: gluconeogenesis, suppressing immune system responses and carbohydrate metabolism.
One of the symptoms of cortisone reductase deficiency is hyperandrogenism, resulting from activation of the Hypothalamic–pituitary–adrenal axis. The deficiency has been known to exhibit symptoms of other disorders such as Polycystic Ovary Syndrome in women. Cortisone Reductase Deficiency alone has been reported in fewer than ten cases in total, all but one case were women. Elevated activity of 11β-HSD1 can lead to obesity or Type II Diabetes, because of the role of cortisol in carbohydrate metabolism and gluconeogenesis.

11α-Hydroxyprogesterone (11α-OHP), or 11α-hydroxypregn-4-ene-3,20-dione is an endogenous steroid and metabolite of progesterone. It is a weak antiandrogen, and is devoid of androgenic, estrogenic, and progestogenic activity.
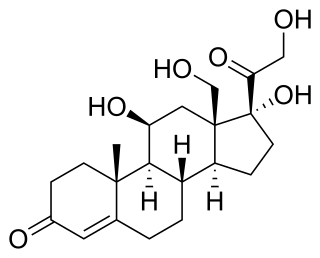
18-Hydroxycortisol is an endogenous steroid, a metabolite of cortisol.

18-Oxocortisol is an endogenous steroid, a metabolite of cortisol.

















