
Adolph Wilhelm Hermann Kolbe was a seminal contributor in the birth of modern organic chemistry. He was a Professor at Marburg and Leipzig. Kolbe coined the term synthesis and contributed to the philosophical demise of vitalism through synthesis of the organic substance acetic acid from carbon disulfide, and also contributed to the development of structural theory. This was done via modifications to the idea of "radicals" and accurate prediction of the existence of secondary and tertiary alcohols, and to the emerging array of organic reactions through his Kolbe electrolysis of carboxylate salts, the Kolbe-Schmitt reaction in the preparation of aspirin and the Kolbe nitrile synthesis. After studies with Wöhler and Bunsen, Kolbe was involved with the early internationalization of chemistry through overseas work in London, and rose through the ranks of his field to edit the Journal für Praktische Chemie. As such, he was elected to the Royal Swedish Academy of Sciences won the Royal Society of London's Davy Medal in the year of his death. Despite these accomplishments and his training a storied next generation of chemists, Kolbe is remembered for editing the Journal for more than a decade, where his rejection of Kekulé's structure of benzene, van't Hoff's theory on the origin of chirality and von Baeyer's reforms of nomenclature were personally critical and linguistically violent. Kolbe died of a heart attack in Leipzig at age 68, six years after the death of his wife, Charlotte. He was survived by four children.

Otto Wallach was a German chemist and recipient of the 1910 Nobel Prize in Chemistry for his work on alicyclic compounds.

Robert Burns Woodward was an American organic chemist. He is considered by many to be the preeminent organic chemist of the twentieth century, having made many key contributions to the subject, especially in the synthesis of complex natural products and the determination of their molecular structure. He also worked closely with Roald Hoffmann on theoretical studies of chemical reactions. He was awarded the Nobel Prize in Chemistry in 1965.

Adolf Otto Reinhold Windaus was a German chemist who won a Nobel Prize in Chemistry in 1928 for his work on sterols and their relation to vitamins. He was the doctoral advisor of Adolf Butenandt who also won a Nobel Prize in Chemistry in 1939.

Hermann Staudinger was a German organic chemist who demonstrated the existence of macromolecules, which he characterized as polymers. For this work he received the 1953 Nobel Prize in Chemistry.

Johann Friedrich Wilhelm Adolf von Baeyer was a German chemist who synthesised indigo, developed a nomenclature for cyclic compounds. He was ennobled in the Kingdom of Bavaria in 1885 and was the 1905 recipient of the Nobel Prize in Chemistry.

Hans Fischer was a German organic chemist and the recipient of the 1930 Nobel Prize for Chemistry "for his researches into the constitution of haemin and chlorophyll and especially for his synthesis of haemin."
Ernst Otto Fischer was a German chemist who won the Nobel Prize for pioneering work in the area of organometallic chemistry.

Wilhelm Traube was a German chemist.
The Wulff–Dötz reaction (also known as the Dötz reaction or the benzannulation reaction of the Fischer carbene complexes) is the chemical reaction of an aromatic or vinylic alkoxy pentacarbonyl chromium carbene complex with an alkyne and carbon monoxide to give a Cr(CO)3-coordinated substituted phenol. Several reviews have been published. It is named after the German chemist Karl Heinz Dötz (b. 1943) and the American chemist William D. Wulff (b. 1949) at Michigan State University. The reaction was first discovered by Karl Dötz and was extensively developed by his group and W. Wulff's group. They subsequently share the name of the reaction.
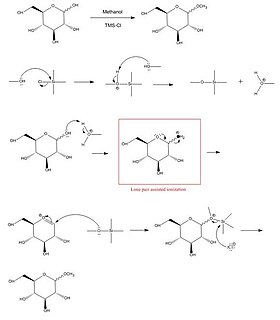
Fischer glycosidation refers to the formation of a glycoside by the reaction of an aldose or ketose with an alcohol in the presence of an acid catalyst. The reaction is named after the German chemist, Emil Fischer, winner of the Nobel Prize in chemistry, 1902, who developed this method between 1893 and 1895.
Otto Roelen was a German chemist.

Johann Peter Griess was an industrial chemist and an early pioneer of organic chemistry.

Ludwig Gattermann was a German chemist who contributed significantly to both organic and inorganic chemistry.

Friedrich Asinger was an Austrian chemist and professor for Technical Chemistry. He is well known for his development of a multi-component reaction, the Asinger reaction for the synthesis of 3-thiazolines.
Alexander J. Fatiadi is a chemist.
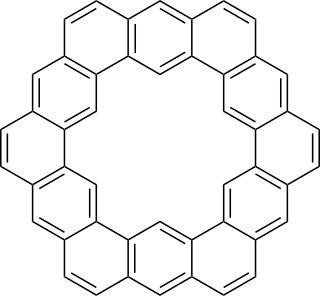
Kekulene is a polycyclic aromatic hydrocarbon and a circulene with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule.
Harry Laurence Anderson, FRS is a British chemist in the Department of Chemistry, University of Oxford. He is well known for his contributions in the syntheses of supramolecular systems and exploration of the extraordinary physical properties of large pi-conjugated systems. He is a Professor of Chemistry at Keble College, Oxford.

Géza Gusztáv Zemplén, Ph.D. was a notable Hungarian chemist, organic chemist, professor, and chemistry author. He was a recipient of the Kossuth Prize, a member of the Hungarian Academy of Sciences, and was the brother of Professor Győző Zemplén. His major field of research was structural chemistry and biochemistry including the synthesis of naturally occurring flavonoid-glycosides.




























