
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.

Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge.

Redox is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction.

Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions.
In chemistry, a reducing agent is a chemical species that "donates" an electron to an electron recipient.
Digestion is the breakdown of carbohydrates to yield an energy-rich compound called ATP. The production of ATP is achieved through the oxidation of glucose molecules. In oxidation, the electrons are stripped from a glucose molecule to reduce NAD+ and FAD. NAD+ and FAD possess a high energy potential to drive the production of ATP in the electron transport chain. ATP production occurs in the mitochondria of the cell. There are two methods of producing ATP: aerobic and anaerobic. In aerobic respiration, oxygen is required. Using oxygen increases ATP production from 4 ATP molecules to about 30 ATP molecules. In anaerobic respiration, oxygen is not required. When oxygen is absent, the generation of ATP continues through fermentation. There are two types of fermentation: alcohol fermentation and lactic acid fermentation.

In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds.
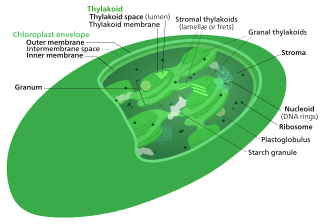
The Calvin cycle, light-independent reactions, bio synthetic phase, dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into glucose. The Calvin cycle is present in all photosynthetic eukaryotes and also many photosynthetic bacteria. In plants, these reactions occur in the stroma, the fluid-filled region of a chloroplast outside the thylakoid membranes. These reactions take the products of light-dependent reactions and perform further chemical processes on them. The Calvin cycle uses the chemical energy of ATP and reducing power of NADPH from the light dependent reactions to produce sugars for the plant to use. These substrates are used in a series of reduction-oxidation (redox) reactions to produce sugars in a step-wise process; there is no direct reaction that converts several molecules of CO2 to a sugar. There are three phases to the light-independent reactions, collectively called the Calvin cycle: carboxylation, reduction reactions, and ribulose 1,5-bisphosphate (RuBP) regeneration.
In chemistry, the valence or valency of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemical bonds that each atom of a given chemical element typically forms. Double bonds are considered to be two bonds, triple bonds to be three, quadruple bonds to be four, quintuple bonds to be five and sextuple bonds to be six. In most compounds, the valence of hydrogen is 1, of oxygen is 2, of nitrogen is 3, and of carbon is 4. Valence is not to be confused with the related concepts of the coordination number, the oxidation state, or the number of valence electrons for a given atom.

In chemistry, a formal charge, in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity. In simple terms, formal charge is the difference between the number of valence electrons of an atom in a neutral free state and the number assigned to that atom in a Lewis structure. When determining the best Lewis structure for a molecule, the structure is chosen such that the formal charge on each of the atoms is as close to zero as possible.
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called comproportionation, also known as symproportionation.

Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen. Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation:
Redox potential is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively. Redox potential is expressed in volts (V). Each species has its own intrinsic redox potential; for example, the more positive the reduction potential, the greater the species' affinity for electrons and tendency to be reduced.

In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.

An ion is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.

In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.