Related Research Articles

A protease is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism, and cell signaling.

Histone methyltransferases (HMT) are histone-modifying enzymes, that catalyze the transfer of one, two, or three methyl groups to lysine and arginine residues of histone proteins. The attachment of methyl groups occurs predominantly at specific lysine or arginine residues on histones H3 and H4. Two major types of histone methyltranferases exist, lysine-specific and arginine-specific. In both types of histone methyltransferases, S-Adenosyl methionine (SAM) serves as a cofactor and methyl donor group.
The genomic DNA of eukaryotes associates with histones to form chromatin. The level of chromatin compaction depends heavily on histone methylation and other post-translational modifications of histones. Histone methylation is a principal epigenetic modification of chromatin that determines gene expression, genomic stability, stem cell maturation, cell lineage development, genetic imprinting, DNA methylation, and cell mitosis.

Histone acetyltransferases (HATs) are enzymes that acetylate conserved lysine amino acids on histone proteins by transferring an acetyl group from acetyl-CoA to form ε-N-acetyllysine. DNA is wrapped around histones, and, by transferring an acetyl group to the histones, genes can be turned on and off. In general, histone acetylation increases gene expression.
A metalloproteinase, or metalloprotease, is any protease enzyme whose catalytic mechanism involves a metal. An example is ADAM12 which plays a significant role in the fusion of muscle cells during embryo development, in a process known as myogenesis.
The kinin–kallikrein system or simply kinin system is a poorly understood hormonal system with limited available research. It consists of blood proteins that play a role in inflammation, blood pressure control, coagulation and pain. Its important mediators bradykinin and kallidin are vasodilators and act on many cell types. Clinical symptoms include marked weakness, tachycardia, fever, leukocytosis and acceleration of ESR.
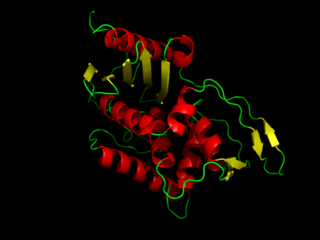
DD-transpeptidase is a bacterial enzyme that catalyzes the transfer of the R-L-αα-D-alanyl moiety of R-L-αα-D-alanyl-D-alanine carbonyl donors to the γ-OH of their active-site serine and from this to a final acceptor. It is involved in bacterial cell wall biosynthesis, namely, the transpeptidation that crosslinks the peptide side chains of peptidoglycan strands.
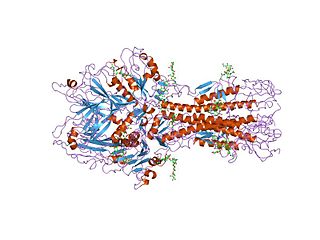
Hemagglutinin esterase (HEs) is a glycoprotein that certain enveloped viruses possess and use as an invading mechanism. HEs helps in the attachment and destruction of certain sialic acid receptors that are found on the host cell surface. Viruses that possess HEs include influenza C virus, toroviruses, and coronaviruses of the subgenus Embecovirus. HEs is a dimer transmembrane protein consisting of two monomers, each monomer is made of three domains. The three domains are: membrane fusion, esterase, and receptor binding domains.

A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes. An acid-base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence.

An ATP-binding motif is a 250-residue sequence within an ATP-binding protein’s primary structure. The binding motif is associated with a protein’s structure and/or function. ATP is a molecule of energy, and can be a coenzyme, involved in a number of biological reactions. ATP is proficient at interacting with other molecules through a binding site. The ATP binding site is the environment in which ATP catalytically actives the enzyme and, as a result, is hydrolyzed to ADP. The binding of ATP causes a conformational change to the enzyme it is interacting with.
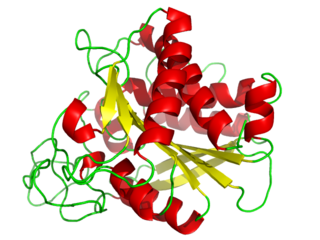
A carboxypeptidase is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed.
Proprotein convertases (PPCs) are a family of proteins that activate other proteins. Many proteins are inactive when they are first synthesized, because they contain chains of amino acids that block their activity. Proprotein convertases remove those chains and activate the protein. The prototypical proprotein convertase is furin. Proprotein convertases have medical significance, because they are involved in many important biological processes, such as cholesterol synthesis. Compounds called proprotein convertase inhibitors can block their action, and block the target proteins from becoming active. Many proprotein convertases, especially furin and PACE4, are involved in pathological processes such as viral infection, inflammation, hypercholesterolemia, and cancer, and have been postulated as therapeutic targets for some of these diseases.
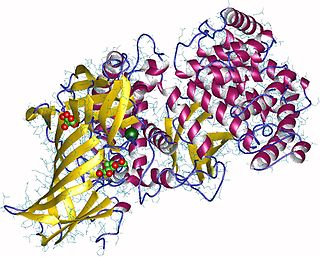
Aminopeptidases are enzymes that catalyze the cleavage of amino acids from the amino terminus (N-terminus) of proteins or peptides (exopeptidases). They are widely distributed throughout the animal and plant kingdoms and are found in many subcellular organelles, in cytosol, and as membrane components. Aminopeptidases are used in essential cellular functions. Many, but not all, of these peptidases are zinc metalloenzymes.

Aspartoacylase is a hydrolytic enzyme that in humans is encoded by the ASPA gene. ASPA catalyzes the deacylation of N-acetyl-l-aspartate (N-acetylaspartate) into aspartate and acetate. It is a zinc-dependent hydrolase that promotes the deprotonation of water to use as a nucleophile in a mechanism analogous to many other zinc-dependent hydrolases. It is most commonly found in the brain, where it controls the levels of N-acetyl-l-aspartate. Mutations that result in loss of aspartoacylase activity are associated with Canavan disease, a rare autosomal recessive neurodegenerative disease.
Protein metabolism denotes the various biochemical processes responsible for the synthesis of proteins and amino acids (anabolism), and the breakdown of proteins by catabolism.

Carboxypeptidase A usually refers to the pancreatic exopeptidase that hydrolyzes peptide bonds of C-terminal residues with aromatic or aliphatic side-chains. Most scientists in the field now refer to this enzyme as CPA1, and to a related pancreatic carboxypeptidase as CPA2.
The discovery of an orally inactive peptide from snake venom established the important role of angiotensin converting enzyme (ACE) inhibitors in regulating blood pressure. This led to the development of captopril, the first ACE inhibitor. When the adverse effects of captopril became apparent new derivates were designed. Then after the discovery of two active sites of ACE: N-domain and C-domain, the development of domain-specific ACE inhibitors began.

The enzyme Acid-Induced Arginine Decarboxylase (AdiA), also commonly referred to as arginine decarboxylase, catalyzes the conversion of L-arginine into agmatine and carbon dioxide. The process consumes a proton in the decarboxylation and employs a pyridoxal-5'-phosphate (PLP) cofactor, similar to other enzymes involved in amino acid metabolism, such as ornithine decarboxylase and glutamine decarboxylase. It is found in bacteria and virus, though most research has so far focused on forms of the enzyme in bacteria. During the AdiA catalyzed decarboxylation of arginine, the necessary proton is consumed from the cell cytoplasm which helps to prevent the over-accumulation of protons inside the cell and serves to increase the intracellular pH. Arginine decarboxylase is part of an enzymatic system in Escherichia coli, Salmonella Typhimurium, and methane-producing bacteria Methanococcus jannaschii that makes these organisms acid resistant and allows them to survive under highly acidic medium.

Carboxypeptidase N catalytic chain is an enzyme that in humans is encoded by the CPN1 gene.
Peptidyl-dipeptidase Dcp (EC 3.4.15.5, dipeptidyl carboxypeptidase (Dcp), dipeptidyl carboxypeptidase) is a metalloenzyme found in the cytoplasm of bacterium E. Coli responsible for the C-terminal cleavage of a variety of dipeptides and unprotected larger peptide chains. The enzyme does not hydrolyze bonds in which P1' is Proline, or both P1 and P1' are Glycine. Dcp consists of 680 amino acid residues that form into a single active monomer which aids in the intracellular degradation of peptides. Dcp coordinates to divalent zinc which sits in the pocket of the active site and is composed of four subsites: S1’, S1, S2, and S3, each subsite attracts certain amino acids at a specific position on the substrate enhancing the selectivity of the enzyme. The four subsites detect and bind different amino acid types on the substrate peptide in the P1 and P2 positions. Some metallic divalent cations such as Ni+2, Cu+2, and Zn+2 inhibit the function of the enzyme around 90%, whereas other cations such as Mn+2, Ca+2, Mg+2, and Co+2 have slight catalyzing properties, and increase the function by around 20%. Basic amino acids such as Arginine bind preferably at the S1 site, the S2 site sits deeper in the enzyme therefore is restricted to bind hydrophobic amino acids with phenylalanine in the P2 position. Dcp is divided into two subdomains (I, and II), which are the two sides of the clam shell-like structure and has a deep inner cavity where a pair of histidine residues bind to the catalytic zinc ion in the active site. Peptidyl-Dipeptidase Dcp is classified like Angiotensin-I converting enzyme (ACE) which is also a carboxypeptidase involved in blood pressure regulation, but due to structural differences and peptidase activity between these two enzymes they had to be examined separately. ACE has endopeptidase activity, whereas Dcp strictly has exopeptidase activity based on its cytoplasmic location and therefore their mechanisms of action are differentiated. Another difference between these enzymes is that the activity of Peptidyl-Dipeptidase Dcp is not enhanced in the presence of chloride anions, whereas chloride enhances ACE activity.
Protein methylation is a type of post-translational modification featuring the addition of methyl groups to proteins. It can occur on the nitrogen-containing side-chains of arginine and lysine, but also at the amino- and carboxy-termini of a number of different proteins. In biology, methyltransferases catalyze the methylation process, activated primarily by S-adenosylmethionine. Protein methylation has been most studied in histones, where the transfer of methyl groups from S-adenosyl methionine is catalyzed by histone methyltransferases. Histones that are methylated on certain residues can act epigenetically to repress or activate gene expression.
References
- ↑ Plummer TH, Erdös EG (January 1981). "Human plasma carboxypeptidase N". Proteolytic Enzymes, Part C. Methods in Enzymology. Vol. 80 Pt C. Academic Press. pp. 442–449. doi:10.1016/s0076-6879(81)80038-9. PMID 7341915.
- ↑ Levin Y, Skidgel RA, Erdös EG (August 1982). "Isolation and characterization of the subunits of human plasma carboxypeptidase N (kininase i)". Proceedings of the National Academy of Sciences of the United States of America. 79 (15): 4618–4622. Bibcode:1982PNAS...79.4618L. doi: 10.1073/pnas.79.15.4618 . PMC 346726 . PMID 6750606.
- 1 2 3 4 Skidgel RA (August 1988). "Basic carboxypeptidases: regulators of peptide hormone activity". Trends in Pharmacological Sciences. 9 (8): 299–304. doi:10.1016/0165-6147(88)90015-6. PMID 3074547.
- 1 2 "Enzyme Classification". iubmb.qmul.ac.uk. Retrieved 2022-09-20.
- ↑ Nomenclature Committee (March 2019). "The Enzyme List - Class 3 - Hydrolases" (PDF). International Union of Biochemistry and Molecular Biology.
{{cite web}}: CS1 maint: url-status (link) - ↑ "CPN1 orthologs". NCBI. Retrieved 2022-09-21.
- 1 2 3 4 5 6 7 8 Skidgel RA, Erdös EG (December 2007). "Structure and function of human plasma carboxypeptidase N, the anaphylatoxin inactivator". International Immunopharmacology. Special Issue in Honor of Tony Hugli. 7 (14): 1888–1899. doi:10.1016/j.intimp.2007.07.014. PMC 2679228 . PMID 18039526.
- 1 2 3 4 Keil C, Maskos K, Than M, Hoopes JT, Huber R, Tan F, et al. (February 2007). "Crystal structure of the human carboxypeptidase N (kininase I) catalytic domain". Journal of Molecular Biology. 366 (2): 504–516. doi:10.1016/j.jmb.2006.11.025. PMID 17157876.
- 1 2 Hendriks D, Vingron M, Vriend G, Wang W, Nalis D, Scharpé S (September 1993). "On the specificity of carboxypeptidase N, a comparative study". Biological Chemistry Hoppe-Seyler. 374 (9): 843–849. doi:10.1515/bchm3.1993.374.7-12.843. PMID 8267877.