
Fish farming or pisciculture involves commercial breeding of fish, most often for food, in fish tanks or artificial enclosures such as fish ponds. It is a particular type of aquaculture, which is the controlled cultivation and harvesting of aquatic animals such as fish, crustaceans, molluscs and so on, in natural or pseudo-natural environments. A facility that releases juvenile fish into the wild for recreational fishing or to supplement a species' natural numbers is generally referred to as a fish hatchery. Worldwide, the most important fish species produced in fish farming are carp, catfish, salmon and tilapia.

Orthomyxoviridae is a family of negative-sense RNA viruses. It includes seven genera: Alphainfluenzavirus, Betainfluenzavirus, Gammainfluenzavirus, Deltainfluenzavirus, Isavirus, Thogotovirus, and Quaranjavirus. The first four genera contain viruses that cause influenza in birds and mammals, including humans. Isaviruses infect salmon; the thogotoviruses are arboviruses, infecting vertebrates and invertebrates. The Quaranjaviruses are also arboviruses, infecting vertebrates (birds) and invertebrates (arthropods).

The Atlantic salmon is a species of ray-finned fish in the family Salmonidae. It is the third largest of the Salmonidae, behind Siberian taimen and Pacific Chinook salmon, growing up to a meter in length. Atlantic salmon are found in the northern Atlantic Ocean and in rivers that flow into it. Most populations are anadromous, hatching in streams and rivers but moving out to sea as they grow where they mature, after which the adults seasonally move upstream again to spawn.
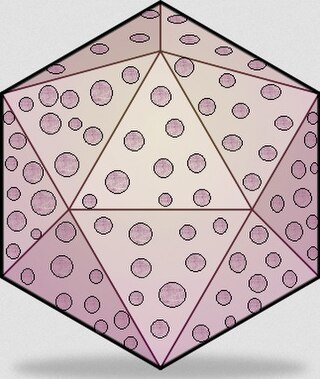
Chicken anemia virus, or CAV, is currently a member of the Anelloviridae family which is found worldwide. The virus only affects chickens. CAV is a non-enveloped icosahedral single stranded DNA virus, which causes bone marrow atrophy, anemia, and severe immunosuppression. Clinical signs of CAV infection are predominantly found in young chicks due to vertical transmission from the breeder hens whose maternal antibodies have not yet formed following exposure. Clinical disease is rare today because of the widespread practice of vaccinating breeders, but the subclinical form of the disease—which normally affects birds more than two weeks of age following horizontal transmission of the virus via the fecal–oral route—is ubiquitous. The virus is very resistant in the environment, making elimination very difficult.

Sea lice are copepods of the family Caligidae within the order Siphonostomatoida. They are marine ectoparasites that feed on the mucus, epidermal tissue, and blood of host fish. The roughly 559 species in 37 genera include around 162 Lepeophtheirus and 268 Caligus species.
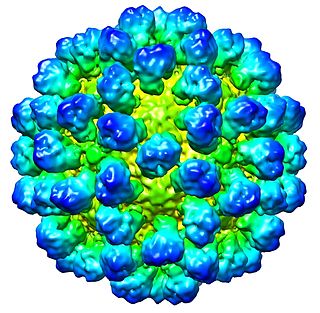
Rabbit hemorrhagic disease (RHD), also known as viral hemorrhagic disease (VHD), is a highly infectious and lethal form of viral hepatitis that affects European rabbits. Some viral strains also affect hares and cottontail rabbits. Mortality rates generally range from 70 to 100 percent. The disease is caused by strains of rabbit hemorrhagic disease virus (RHDV), a lagovirus in the family Caliciviridae.

Viral hemorrhagic septicemia (VHS) is a deadly infectious fish disease caused by Viral hemorrhagic septicemia virus. It afflicts over 50 species of freshwater and marine fish in several parts of the Northern Hemisphere. Different strains of the virus occur in different regions, and affect different species. There are no signs that the disease affects human health. VHS is also known as Egtved disease, and the virus as Egtved virus.
Infectious hematopoietic necrosis virus (IHNV), is a negative-sense single-stranded, bullet-shaped RNA virus that is a member of the Rhabdoviridae family, and from the genus Novirhabdovirus. It causes the disease known as infectious hematopoietic necrosis in salmonid fish such as trout and salmon. The disease may be referred to by a number of other names such as Chinook salmon disease, Coleman disease, Columbia River sockeye disease, Cultus Lake virus disease, Oregon sockeye disease, Sacramento River Chinook disease and sockeye salmon viral disease. IHNV is commonly found in the Pacific Coast of Canada and the United States, and has also been found in Europe and Japan. The first reported epidemics of IHNV occurred in the United States at the Washington and the Oregon fish hatcheries during the 1950s. IHNV is transmitted following shedding of the virus in the feces, urine, sexual fluids, and external mucus and by direct contact or close contact with the surrounding water. The virus gains entry into fish at the base of the fins.

Maine, in the United States, has a tradition of having a large fishing and lobster industry. However today some of that industry has switched to salmon farming or aquaculture. Of late aquaculturists in Maine are most concerned about the outbreak of Infectious Salmon anemia(ISA) in the Bay of Fundy, New Brunswick. The Canadian and US salmon raising industries in the bay are geographically near one another and are therefore ecologically integrated. Machias Bay, which is 50 miles west of the Bay of Fundy, is also a location of salmon raising in Maine close to the Bay of Fundy.

The salmon louse is a species of copepod in the genus Lepeophtheirus. It is a sea louse, a parasite living mostly on salmon, particularly on Pacific and Atlantic salmon and sea trout, but is also sometimes found on the three-spined stickleback. It feeds on the mucus, skin and blood of the fish. Once detached, they can be blown by wind across the surface of the sea, like plankton. When they encounter a suitable marine fish host, they adhere themselves to the skin, fins, or gills of the fish, and feed on the mucus or skin. Sea lice only affect fish and are not harmful to humans.

Foot-and-mouth disease (FMD) or hoof-and-mouth disease (HMD) is an infectious and sometimes fatal viral disease that affects cloven-hoofed animals, including domestic and wild bovids. The virus causes a high fever lasting two to six days, followed by blisters inside the mouth and near the hoof that may rupture and cause lameness.

The aquaculture of salmonids is the farming and harvesting of salmonid fish under controlled conditions for both commercial and recreational purposes. Salmonids, along with carp and tilapia, are the three most important fish groups in aquaculture. The most commonly commercially farmed salmonid is the Atlantic salmon.

Like humans and other animals, fish suffer from diseases and parasites. Fish defences against disease are specific and non-specific. Non-specific defences include skin and scales, as well as the mucus layer secreted by the epidermis that traps microorganisms and inhibits their growth. If pathogens breach these defences, fish can develop inflammatory responses that increase the flow of blood to infected areas and deliver white blood cells that attempt to destroy the pathogens.

Diseases and parasites in salmon, trout and other salmon-like fishes of the family Salmonidae are also found in other fish species. The life cycle of many salmonids is anadromous, so such fish are exposed to parasites in fresh water, brackish water and saline water.

Infectious pancreatic necrosis virus (IPNV) is a double-stranded RNA virus from the family Birnaviridae, in the genus Aquabirnavirus. Causing the highly infectious disease Infectious pancreatic necrosis, the virus primarily affects young salmonids resulting in high mortality, occasionally surpassing 90 percent in the early stages. IPNV or IPNV-like viruses have been isolated worldwide from at least 32 families of saltwater and freshwater salmonids and non-salmonids fish including salmon, flatfish, pike, eels and others. Other aquatic organisms infected include 11 molluscs and 4 species of crustaceans. Due to its wide host range and high mortality, the virus is of great concern to global aquaculture. In addition to persistence in hosts, IPNV is also perpetual in the environment, surviving across a range of conditions and capable of infecting fish with as little as 101TCID50/ml of the virus. Found in Europe, North America, South America, Africa, Asia, and Australia, the virus has led to significant losses in the mariculture of Atlantic salmon, brook trout, and rainbow trout.
Piscirickettsia salmonis is the bacterial causative agent of piscirickettsiosis, an epizootic disease in salmonid fishes. It has a major impact on salmon populations, with a mortality rate of up to 90% in some species. The type strain, LF-89, is from Chile, but multiple strains exist, and some are more virulent than others. P. salmonis and piscrickettsiosis are present in various geographic regions from Europe to Oceania to South America, but the Chilean salmon farming industry has been particularly hard-hit. Different strategies of controlling the disease and farm-to-farm spread have been the subject of much research, but a significant amount is still unknown.
Tilapia tilapinevirus, or Tilapia lake virus (TiLV), is a negative-strand RNA virus that infects both wild and aquacultured populations of tilapia. It is the only species in the monotypic genus Tilapinevirus, which in turn is the only genus in the family Amnoonviridae. Thus far it has been recorded in various regions across Asia, Africa, and South America. The virus was first discovered and identified in 2014 when the Sea of Galilee in Israel experienced a major noticeable decline in tilapia catch quantities.
Salmon Pancreas disease is caused by a species of Salmonid Alphavirus (SAV) called Salmon pancreas disease virus (SPDV). The virus was first described in 1976 in Scotland and in 1989 in Norway. It affects farmed Atlantic salmon caused by Marine SAV2 and SAV3 and has also been identified in Rainbow trout in the seawater phase caused by SAV2 where the disease is commonly referred to as Sleeping Disease (SD).
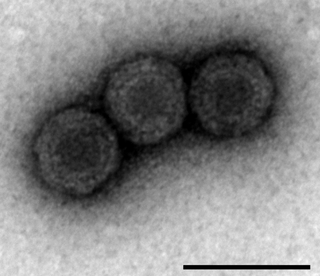
Piscine orthoreovirus (PRV) is a species in the genus Orthoreovirus that infects fish exclusively, PRV was first discovered in 2010 in farmed Atlantic salmon exhibiting Heart and Skeletal Muscle Inflammation (HSMI) and has been found present at higher concentration in fish with various diseases. These diseases include HSMI, jaundice syndrome, proliferative darkening syndrome and erythrocytic body inclusion syndrome. PRV is thought to mainly affect aquacultured and maricultured fish stocks, and recent research has been focused around the susceptibility of wild stock. However, whether PRV is virulent with respect to HSMI remains a topic of debate. PRV has been in the public eye mostly due to a potential linkage to farmed Atlantic Salmon exhibiting HSMI. Public concern has been raised regarding the possibility of open ocean-net farms transmitting PRV to wild salmon populations and being a factor in declining populations. PRV has not been confirmed to be pathogenic in wild salmon stocks.
Mycobacteroides salmoniphilum is a species of bacteria from the phylum Actinomycetota belonging to the genus Mycobacteroides. It was first identified as the causative agent of mycobacteriosis in chinook salmon and steelhead trout, but has since been found to cause disease in Atlantic cod, Atlantic salmon, burbot, coho salmon, freshwater ornamental fish, and Russian sturgeon. It has also been isolated from tap water. It is not known to infect humans. M. salmoniphilum is susceptible to amikacin.















