The data values of standard electrode potentials (E°) are given in the table below, in volts relative to the standard hydrogen electrode, and are for the following conditions:

The square metre or square meter is the unit of area in the International System of Units (SI) with symbol m2. It is the area of a square with sides one metre in length.

The Honda CR-X, originally launched as the Honda Ballade Sports CR-X in Japan, is a front-wheel-drive sport compact car manufactured by Honda from 1983 until 1991. The first generation CRX was marketed in some regions outside Japan as the Honda Civic CRX. Although there are many supposed definitions for the acronym CR-X, the most widely accepted is "Civic Renaissance Experimental".
An oxyanion, or oxoanion, is an ion with the generic formula A
xOz−
y. Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound H
zA
xO
y. The structures of condensed oxyanions can be rationalized in terms of AOn polyhedral units with sharing of corners or edges between polyhedra. The oxyanions adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology.

Chromium(III) chloride (also called chromic chloride) describes any of several chemical compounds with the formula CrCl3 · xH2O, where x can be 0, 5, and 6. The anhydrous compound with the formula CrCl3 is a violet solid. The most common form of the trichloride is the dark green hexahydrate, CrCl3 · 6 H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.
The equivalent carbon content concept is used on ferrous materials, typically steel and cast iron, to determine various properties of the alloy when more than just carbon is used as an alloyant, which is typical. The idea is to convert the percentage of alloying elements other than carbon to the equivalent carbon percentage, because the iron-carbon phases are better understood than other iron-alloy phases. Most commonly this concept is used in welding, but it is also used when heat treating and casting cast iron.

Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions, bright orange when wet and which dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser and a carcinogen.
Naturally occurring manganese (25Mn) is composed of one stable isotope, 55Mn. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3.7 million years, 54Mn with a half-life of 312.3 days, and 52Mn with a half-life of 5.591 days. All of the remaining radioactive isotopes have half-lives that are less than 3 hours and the majority of these have half-lives that are less than a minute, but only 45Mn has an unknown half-life. The least stable is 44Mn with a half-life shorter than 105 nanoseconds. This element also has 3 meta states.
Naturally occurring chromium (24Cr) is composed of four stable isotopes; 50Cr, 52Cr, 53Cr, and 54Cr with 52Cr being the most abundant (83.789% natural abundance). 50Cr is suspected of decaying by β+β+ to 50Ti with a half-life of (more than) 1.8×1017 years. Twenty-two radioisotopes, all of which are entirely synthetic, have been characterized with the most stable being 51Cr with a half-life of 27.7 days. All of the remaining radioactive isotopes have half-lives that are less than 24 hours and the majority of these have half-lives that are less than 1 minute, the least stable being 66Cr with a half-life of 10 milliseconds. This element also has 2 meta states, 45mCr, the more stable one, and 59mCr, the least stable isotope or isomer.

Chromium(II) chloride describes inorganic compounds with the formula CrCl2(H2O)n. The anhydrous solid is white when pure, however commercial samples are often grey or green; it is hygroscopic and readily dissolves in water to give bright blue air-sensitive solutions of the tetrahydrate Cr(H2O)4Cl2. Chromium(II) chloride has no commercial uses but is used on a laboratory-scale for the synthesis of other chromium complexes.

Caesium chromate or cesium chromate is an inorganic compound with the formula Cs2CrO4. It is a yellow crystalline solid that is the caesium salt of chromic acid, and it crystallises in the orthorhombic system.
Chromium(IV) chloride (CrCl4) is an unstable chromium compound. It is generated by combining chromium(III) chloride and chlorine gas at elevated temperatures, but reverts to those substances at room temperature.
Iron(III) chromate is the iron(III) salt of chromic acid with the chemical formula Fe2(CrO4)3.

Silicon monosulfide is a chemical compound of silicon and sulfur. The chemical formula is SiS. Molecular SiS has been detected at high temperature in the gas phase. The gas phase molecule has an Si-S bondlength of 192.93 pm, this compares to the normal single bond length of 216 pm, and is shorter than the Si=S bond length of around 201 pm reported in an organosilanethione. Historically a pale yellow-red amorphous solid compound has been reported. The behavior of silicon can be contrasted with germanium which forms a stable solid monosulfide.

Digermane is an inorganic compound with the chemical formula Ge2H6. One of the few hydrides of germanium, it is a colourless liquid. Its molecular geometry is similar to ethane.
Chromium(II) silicide or chromium disilicide is an inorganic compound of chromium and silicon. Its chemical formula is CrSi2. It is a p-type thermoelectric semiconductor with an indirect bandgap of 0.35 eV.
Calcium monosilicide (CaSi) is an inorganic compound, a silicide of calcium. It can be prepared by reacting elemental calcium and silicon at temperatures above 1000 °C. It is a Zintl phase, where silicon has oxidation state −2 and covalence 2.
Gallium(I) oxide, digallium monoxide or gallium suboxide is an inorganic compound with the formula Ga2O.
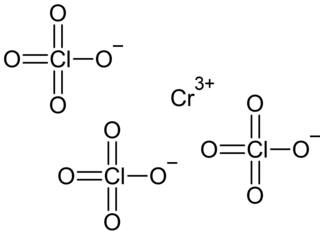
Chromium(III) perchlorate is an inorganic compound with the chemical formula Cr(ClO4)3. It's hexahydrate Cr(ClO4)3·6H2O is a cyan solid that dissolves in water.








