A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups. Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (η5-) bonding mode. The metal–cyclopentadienyl interaction is typically drawn as a single line from the metal center to the center of the Cp ring.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic red-brown solids. The soluble trihydrated (n = 3) salt is the usual compound of commerce. It is widely used to prepare compounds used in homogeneous catalysis.

Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures.
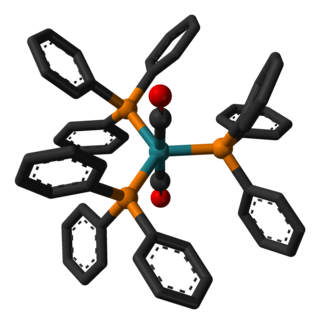
Dicarbonyltris(triphenylphosphine)ruthenium(0) or Roper's complex is a ruthenium metal carbonyl. In it, two carbon monoxide ligands and three triphenylphosphine ligands are coordinated to a central ruthenium(0) center.

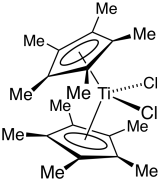
Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.

Dicarbonylbis(cyclopentadienyl)titanium is the chemical compound with the formula (η5-C5H5)2Ti(CO)2, abbreviated Cp2Ti(CO)2. This maroon-coloured, air-sensitive species is soluble in aliphatic and aromatic solvents. It has been used for the deoxygenation of sulfoxides, reductive coupling of aromatic aldehydes and reduction of aldehydes.

Organozirconium chemistry is the science of exploring the properties, structure, and reactivity of organozirconium compounds, which are organometallic compounds containing chemical bonds between carbon and zirconium. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.
Zirconocene dichloride is an organozirconium compound composed of a zirconium central atom, with two cyclopentadienyl and two chloro ligands. It is a colourless diamagnetic solid that is somewhat stable in air.

Pentamethylcyclopentadienyl iridium dichloride dimer is an organometallic compound with the formula [(C5(CH3)5IrCl2)]2, commonly abbreviated [Cp*IrCl2]2 This bright orange air-stable diamagnetic solid is a reagent in organometallic chemistry.
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.
In organometallic chemistry, bent metallocenes are a subset of metallocenes. In bent metallocenes, the ring systems coordinated to the metal are not parallel, but are tilted at an angle. A common example of a bent metallocene is Cp2TiCl2. Several reagents and much research is based on bent metallocenes.

Titanocene pentasulfide is the organotitanium compound with the formula (C5H5)2TiS5, commonly abbreviated as Cp2TiS5. This metallocene exists as a bright red solid that is soluble in organic solvents. It is of academic interest as a precursor to unusual allotropes of elemental sulfur as well as some related inorganic rings.

Pentamethylcyclopentadienyl ruthenium dichloride is an organoruthenium chemistry with the formula [(C5(CH3)5)RuCl2]2, commonly abbreviated [Cp*RuCl2]2. This brown paramagnetic solid is a reagent in organometallic chemistry. It is an unusual example of a compound that exists as isomers that differ in the intermetallic separation, a difference that is manifested in a number of physical properties.

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).

Bis(cyclopentadienyl)titanium(III) chloride, also known as the Nugent–RajanBabu reagent, is the organotitanium compound which exists as a dimer with the formula [(C5H5)2TiCl]2. It is an air sensitive green solid. The complex finds specialized use in synthetic organic chemistry as a single electron reductant.
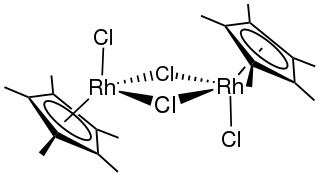
Pentamethylcyclopentadienyl rhodium dichloride dimer is an organometallic compound with the formula [(C5(CH3)5RhCl2)]2, commonly abbreviated [Cp*RhCl2]2 This dark red air-stable diamagnetic solid is a reagent in organometallic chemistry.

(Cyclopentadienyl)titanium trichloride is an organotitanium compound with the formula (C5H5)TiCl3. It is a moisture sensitive orange solid. The compound adopts a piano stool geometry.

Decamethylzirconocene dichloride is an organozirconium compound with the formula Cp*2ZrCl2 (where Cp* is C5(CH3)5, derived from pentamethylcyclopentadiene). It is a pale yellow, moisture sensitive solid that is soluble in nonpolar organic solvents. The complex has been the subject of extensive research. It is a precursor to many other complexes, including the dinitrogen complex [Cp*2Zr]2(N2)3). It is a precatalyst for the polymerization of ethylene and propylene.
Titanocene bis(trimethylsilyl)acetylene is a formally titanium(II) organometallic compound with the formula Ti(C5H5)2C2(Si(CH3)3)2. This complex and its zirconium analogue are often referred to as Rosenthal's reagent, after the first chemist to synthesize it, Uwe Rosenthal. This article will discuss its history, synthesis, structure, reactivity, and applications.