Related Research Articles
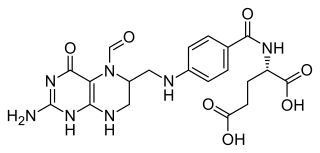
Folinic acid, also known as leucovorin, is a medication used to decrease the toxic effects of methotrexate and pyrimethamine. It is also used in combination with 5-fluorouracil to treat colorectal cancer and pancreatic cancer, may be used to treat folate deficiency that results in anemia, and methanol poisoning. It is taken by mouth, injection into a muscle, or injection into a vein.
Fluorouracil, sold under the brand name Adrucil among others, is a cytotoxic chemotherapy medication used to treat cancer. By intravenous injection it is used for treatment of colorectal cancer, oesophageal cancer, stomach cancer, pancreatic cancer, breast cancer, and cervical cancer. As a cream it is used for actinic keratosis, basal cell carcinoma, and skin warts.
Bevacizumab, sold under the brand name Avastin among others, is a monoclonal antibody medication used to treat a number of types of cancers and a specific eye disease. For cancer, it is given by slow injection into a vein (intravenous) and used for colon cancer, lung cancer, ovarian cancer, glioblastoma, and renal-cell carcinoma. In many of these diseases it is used as a first-line therapy. For age-related macular degeneration it is given by injection into the eye (intravitreal).

Capecitabine, sold under the brand name Xeloda among others, is a anticancer medication used to treat breast cancer, gastric cancer and colorectal cancer. For breast cancer it is often used together with docetaxel. It is taken by mouth.

Oxaliplatin, sold under the brand name Eloxatin among others, is a cancer medication used to treat colorectal cancer. It is given by injection into a vein.

Epirubicin is an anthracycline drug used for chemotherapy. It can be used in combination with other medications to treat breast cancer in patients who have had surgery to remove the tumor. It is marketed by Pfizer under the trade name Ellence in the US and Pharmorubicin or Epirubicin Ebewe elsewhere.
FOLFOX is a chemotherapy regimen for treatment of colorectal cancer, made up of the drugs folinic acid, fluorouracil, and oxaliplatin.

Irinotecan, sold under the brand name Camptosar among others, is an anti-cancer medication used to treat colon cancer and small cell lung cancer. For colon cancer it is used either alone or with fluorouracil. For small cell lung cancer it is used with cisplatin. It is given intravenously.
IFL is a chemotherapy regimen for treatment of certain cancers, consisting of concurrent treatment with irinotecan, leucovorin, and fluorouracil.
Tegafur/uracil is a chemotherapy drug combination used in the treatment of cancer, primarily bowel cancer. It is also called UFT or UFUR.
Aflibercept, sold under the brand names Eylea among others, is a medication used to treat wet macular degeneration and metastatic colorectal cancer. It was developed by Regeneron Pharmaceuticals and is approved in the United States and the European Union.
Ramucirumab is a fully human monoclonal antibody (IgG1) developed for the treatment of solid tumors. This drug was developed by ImClone Systems Inc. It was isolated from a native phage display library from Dyax.
Wafik El-Deiry is an American physician and cancer researcher who is the Associate Dean for Oncologic Sciences at the Warren Alpert Medical School, Brown University, Director of the Cancer Center at Brown University, and the Director of the Joint Program in Cancer Biology at Brown University and its affiliated hospitals. He was previously deputy director of Translational Research at Fox Chase Cancer Center, where he was also co-Leader of the Molecular Therapeutics Program.
FOLFIRINOX is a chemotherapy regimen for treatment of advanced pancreatic cancer. It is made up of the following four drugs:

Atezolizumab, sold under the brand name Tecentriq among others, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma and alveolar soft part sarcoma, but discontinued for use in triple-negative breast cancer (TNBC). It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).
FOLFOXIRI is a chemotherapy regimen for the treatment of advanced colorectal cancer. The role of FOLFOXIRI in colorectal cancer has been reviewed.
Abituzumab is a humanized IgG2 monoclonal antibody (mAb) targeted at CD51 currently in development by Merck KGaA Darmstadt, Germany in an attempt to prevent bone lesion metastases in castration-resistant prostate cancer.
This is a historical timeline of the development and progress of cancer treatments, which includes time of discovery, progress, and approval of the treatments.

Cancer pharmacogenomics is the study of how variances in the genome influences an individual’s response to different cancer drug treatments. It is a subset of the broader field of pharmacogenomics, which is the area of study aimed at understanding how genetic variants influence drug efficacy and toxicity.

Fruquintinib, sold under the brand name Fruzaqla, is an anti-cancer medication used for the treatment of colorectal cancer. Fruquintinib is a kinase inhibitor. It is taken by mouth.
References
- ↑ Chen, K; Gong, Y; Zhang, Q; Shen, Y; Zhou, T (2016). "Efficacy and safety of addition of bevacizumab to FOLFIRI or irinotecan/bolus 5-FU/LV (IFL) in patients with metastatic colorectal cancer: A meta-analysis". Medicine. 95 (46): e5221. doi:10.1097/MD.0000000000005221. PMC 5120901 . PMID 27861344.
- ↑ Tournigand, C; André, T; Achille, E; Lledo, G; Flesh, M; Mery-Mignard, D; Quinaux, E; Couteau, C; Buyse, M; Ganem, G; Landi, B; Colin, P; Louvet, C; de Gramont, A (Jan 15, 2004). "FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study". Journal of Clinical Oncology. 22 (2): 229–37. doi: 10.1200/jco.2004.05.113 . PMID 14657227.
- ↑ Kirstein, M. M.; Lange, A.; Prenzler, A.; Manns, M. P.; Kubicka, S.; Vogel, A. (2014). "Targeted Therapies in Metastatic Colorectal Cancer: A Systematic Review and Assessment of Currently Available Data". The Oncologist. 19 (11): 1156–68. doi:10.1634/theoncologist.2014-0032. PMC 4221380 . PMID 25326159.
- ↑ "Onivyde: EPAR – Product Information" (PDF). European Medicines Agency. 25 October 2016. Archived from the original (PDF) on 16 January 2017. Retrieved 14 January 2017.