
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme.

In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P–O–P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate (Na2H2P2O7) and tetrasodium pyrophosphate (Na4P2O7), among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P–O–P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energy phosphate bond.

Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon.

Nucleotide exchange factors (NEFs) are proteins that stimulate the exchange (replacement) of nucleoside diphosphates for nucleoside triphosphates bound to other proteins.

Nucleoside-diphosphate kinases are enzymes that catalyze the exchange of terminal phosphate between different nucleoside diphosphates (NDP) and triphosphates (NTP) in a reversible manner to produce nucleotide triphosphates. Many NDP serve as acceptor while NTP are donors of phosphate group. The general reaction via ping-pong mechanism is as follows: XDP + YTP ←→ XTP + YDP. NDPK activities maintain an equilibrium between the concentrations of different nucleoside triphosphates such as, for example, when guanosine triphosphate (GTP) produced in the citric acid (Krebs) cycle is converted to adenosine triphosphate (ATP). Other activities include cell proliferation, differentiation and development, signal transduction, G protein-coupled receptor, endocytosis, and gene expression.
The stringent response, also called stringent control, is a stress response of bacteria and plant chloroplasts in reaction to amino-acid starvation, fatty acid limitation, iron limitation, heat shock and other stress conditions. The stringent response is signaled by the alarmone (p)ppGpp, and modulates transcription of up to 1/3 of all genes in the cell. This in turn causes the cell to divert resources away from growth and division and toward amino acid synthesis in order to promote survival until nutrient conditions improve.

Succinyl coenzyme A synthetase is an enzyme that catalyzes the reversible reaction of succinyl-CoA to succinate. The enzyme facilitates the coupling of this reaction to the formation of a nucleoside triphosphate molecule from an inorganic phosphate molecule and a nucleoside diphosphate molecule. It plays a key role as one of the catalysts involved in the citric acid cycle, a central pathway in cellular metabolism, and it is located within the mitochondrial matrix of a cell.

Nucleic acid metabolism is a collective term that refers to the variety of chemical reactions by which nucleic acids are either synthesized or degraded. Nucleic acids are polymers made up of a variety of monomers called nucleotides. Nucleotide synthesis is an anabolic mechanism generally involving the chemical reaction of phosphate, pentose sugar, and a nitrogenous base. Degradation of nucleic acids is a catabolic reaction and the resulting parts of the nucleotides or nucleobases can be salvaged to recreate new nucleotides. Both synthesis and degradation reactions require multiple enzymes to facilitate the event. Defects or deficiencies in these enzymes can lead to a variety of diseases.

Guanosine monophosphate synthetase, also known as GMPS is an enzyme that converts xanthosine monophosphate to guanosine monophosphate.

In molecular biology, adenylosuccinate synthase is an enzyme that plays an important role in purine biosynthesis, by catalysing the guanosine triphosphate (GTP)-dependent conversion of inosine monophosphate (IMP) and aspartic acid to guanosine diphosphate (GDP), phosphate and N(6)-(1,2-dicarboxyethyl)-AMP. Adenylosuccinate synthetase has been characterised from various sources ranging from Escherichia coli to vertebrate tissues. In vertebrates, two isozymes are present: one involved in purine biosynthesis and the other in the purine nucleotide cycle.
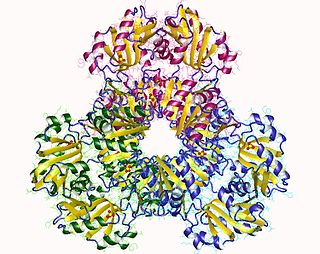
Ribose-phosphate diphosphokinase is an enzyme that converts ribose 5-phosphate into phosphoribosyl pyrophosphate (PRPP). It is classified under EC 2.7.6.1.
In enzymology, an alanine—tRNA ligase is an enzyme that catalyzes the chemical reaction

In enzymology, a succinate—CoA ligase (GDP-forming) is an enzyme that catalyzes the chemical reaction
In enzymology, a guanosine-5'-triphosphate,3'-diphosphate diphosphatase (EC 3.6.1.40) is an enzyme that catalyzes the chemical reaction
The enzyme guanosine-3′,5′-bis(diphosphate) 3′-diphosphatase (EC 3.1.7.2) catalyzes the reaction

In enzymology, an ATP phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a guanosine-triphosphate guanylyltransferase (EC 2.7.7.45) is an enzyme that catalyzes the chemical reaction
In enzymology, a nucleoside-triphosphate-adenylate kinase is an enzyme that catalyzes the chemical reaction
An alarmone is an intracellular signal molecule that is produced in bacteria, chloroplasts, and a slim minority of archaea reacting to harsh environmental factors. They regulate the gene expression at transcription level. Alarmones are produced in high concentrations when harsh environmental factors occur in bacteria and plants, such as lack of amino acids, to produce proteins. Stringent factors take uncharged tRNA and convert it to an alarmone. Guanosine-5'-triphosphate (GTP) is then converted to 5´-diphosphate 3´-diphosphate guanosine (ppGpp), the archetypical alarmone. ppGpp will bind to RNA polymerase β and β´ subunits, changing promoter preference. It will decrease transcription of rRNA and other genes but will increase transcription of genes involved in amino acid biosyntheses and metabolisms involved in famine.
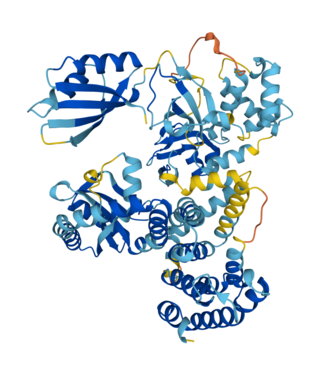
Bifunctional (p)ppGpp synthase/hydrolase SpoT or SpoT is a regulatory enzyme in the RelA/SpoT Homologue (RSH) protein family that synthesizes and hydrolyzes (p)ppGpp to regulate the bacterial stringent response to environmental stressors. SpoT is considered a "long" form RSH protein and is found in many bacteria and plant chloroplasts. SpoT and its homologues have been studied in bacterial model organism E.coli for their role in the production and degradation of (p)ppGpp in the stringent response pathway.












