Anticoagulants, commonly known as blood thinners, are chemical substances that prevent or reduce coagulation of blood, prolonging the clotting time. Some of them occur naturally in blood-eating animals such as leeches and mosquitoes, where they help keep the bite area unclotted long enough for the animal to obtain some blood. As a class of medications, anticoagulants are used in therapy for thrombotic disorders. Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals. Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, and dialysis equipment. They can also be used as rodenticides.
A venous thrombus is a blood clot (thrombus) that forms within a vein. Thrombosis is a term for a blood clot occurring inside a blood vessel. A common type of venous thrombosis is a deep vein thrombosis (DVT), which is a blood clot in the deep veins of the leg. If the thrombus breaks off (embolizes) and flows towards the lungs, it can become a pulmonary embolism (PE), a blood clot in the lungs. This combination is called venous thromboembolism.

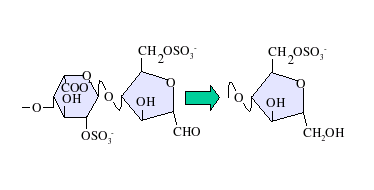
Heparin, also known as unfractionated heparin (UFH), is a medication and naturally occurring glycosaminoglycan. As a medication it is used as an anticoagulant. Specifically it is also used in the treatment of heart attacks and unstable angina. It is given by injection into a vein or under the skin. Other uses include inside test tubes and kidney dialysis machines.
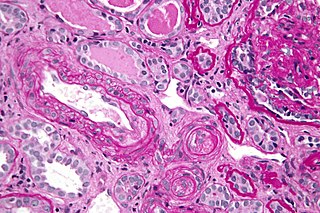
Antiphospholipid syndrome, or antiphospholipid antibody syndrome, is an autoimmune, hypercoagulable state caused by antiphospholipid antibodies. APS provokes blood clots (thrombosis) in both arteries and veins as well as pregnancy-related complications such as miscarriage, stillbirth, preterm delivery, and severe preeclampsia. The diagnostic criteria require one clinical event and two antibody blood tests spaced at least three months apart that confirm the presence of either lupus anticoagulant or anti-β2-glycoprotein-I.

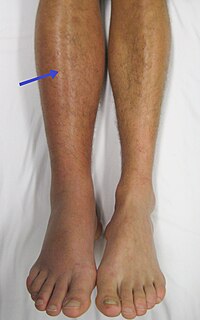
Deep vein thrombosis (DVT) is the formation of a blood clot in a deep vein, most commonly in the legs. Symptoms can include pain, swelling, redness, or warmth of the affected area. About half of cases have no symptoms. Complications can include pulmonary embolism, as a result of detachment of a clot, which travels to the lungs, and post-thrombotic syndrome. Together, DVT and pulmonary embolism are known as venous thromboembolism (VTE).

Heparin-induced thrombocytopenia (HIT) is the development of thrombocytopenia, due to the administration of various forms of heparin, an anticoagulant. HIT predisposes to thrombosis because platelets release microparticles that activate thrombin, thereby leading to thrombosis. When thrombosis is identified the condition is called heparin-induced thrombocytopenia and thrombosis (HITT). HIT is caused by the formation of abnormal antibodies that activate platelets. If someone receiving heparin develops new or worsening thrombosis, or if the platelet count falls, HIT can be confirmed with specific blood tests.

Protein S deficiency is a disorder associated with increased risk of venous thrombosis. Protein S, a vitamin K-dependent physiological anticoagulant, acts as a nonenzymatic cofactor to activate protein C in the degradation of factor Va and factor VIIIa. Decreased (antigen) levels or impaired function of protein S leads to decreased degradation of factor Va and factor VIIIa and an increased propensity to venous thrombosis. Protein S circulates in human plasma in two forms: approximately 60 percent is bound to complement component C4b β-chain while the remaining 40 percent is free, only free protein S has activated protein C cofactor activity
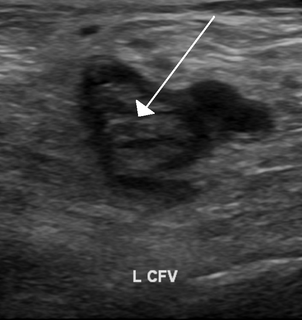
Thrombophilia is an abnormality of blood coagulation that increases the risk of thrombosis. Such abnormalities can be identified in 50% of people who have an episode of thrombosis that was not provoked by other causes. A significant proportion of the population has a detectable abnormality, but most of these only develop thrombosis in the presence of an additional risk factor.

Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.
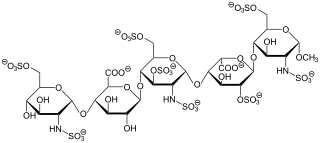
Fondaparinux is an anticoagulant medication chemically related to low molecular weight heparins. It is marketed by GlaxoSmithKline. A generic version developed by Alchemia is marketed within the US by Dr. Reddy's Laboratories.
The prothrombinase complex consists of the serine protein, Factor Xa, and the protein cofactor, Factor Va. The complex assembles on negatively charged phospholipid membranes in the presence of calcium ions. The prothrombinase complex catalyzes the conversion of prothrombin (Factor II), an inactive zymogen, to thrombin (Factor IIa), an active serine protease. The activation of thrombin is a critical reaction in the coagulation cascade, which functions to regulate hemostasis in the body. To produce thrombin, the prothrombinase complex cleaves two peptide bonds in prothrombin, one after Arg271 and the other after Arg320. Although it has been shown that Factor Xa can activate prothrombin when unassociated with the prothrombinase complex, the rate of thrombin formation is severely decreased under such circumstances. The prothrombinase complex can catalyze the activation of prothrombin at a rate 3 x 105-fold faster than can Factor Xa alone. Thus, the prothrombinase complex is required for the efficient production of activated thrombin and also for adequate hemostasis.

Dalteparin is a low molecular weight heparin. It is marketed as Fragmin. Like other low molecular weight heparins, dalteparin is used for prophylaxis or treatment of deep vein thrombosis and pulmonary embolism. It is normally administered by self-injection.
Antithrombin III deficiency is a deficiency of antithrombin III. This deficiency may be inherited or acquired. It is a rare hereditary disorder that generally comes to light when a patient suffers recurrent venous thrombosis and pulmonary embolism, and repetitive intrauterine fetal death (IUFD). Hereditary antithrombin deficiency results in a state of increased coagulation which may lead to venous thrombosis. Inheritance is usually autosomal dominant, though a few recessive cases have been noted. The disorder was first described by Egeberg in 1965. The causes of acquired antithrombin deficiency are easier to find than the hereditary deficiency.
Hypercoagulability in pregnancy is the propensity of pregnant women to develop thrombosis. Pregnancy itself is a factor of hypercoagulability, as a physiologically adaptive mechanism to prevent post partum bleeding. However, when combined with an additional underlying hypercoagulable states, the risk of thrombosis or embolism may become substantial.

Bemiparin is an antithrombotic and belongs to the group of low molecular weight heparins (LMWH).

Reviparin is an antithrombotic and belongs to the group of low molecular weight heparins (LMWH).
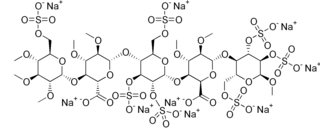
Idraparinux sodium is an anticoagulant medication in development by Sanofi-Aventis.

Direct factor Xa inhibitors ('xabans') are a class of anticoagulant drugs which act directly upon factor X in the coagulation cascade, without using antithrombin as a mediator.
Semuloparin is an experimental antithrombotic being developed by Sanofi-Aventis and belongs to the group of low molecular weight heparins (LMWH). It has completed Phase III clinical trials for the prevention of thromboembolism following various kinds of surgery such as hip replacement, as well as for patients undergoing chemotherapy.
Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases. They inhibit thrombin, a serine protease which affects the coagulation cascade in many ways. DTIs have undergone rapid development since the 90's. With technological advances in genetic engineering the production of recombinant hirudin was made possible which opened the door to this new group of drugs. Before the use of DTIs the therapy and prophylaxis for anticoagulation had stayed the same for over 50 years with the use of heparin derivatives and warfarin which have some well known disadvantages. DTIs are still under development, but the research focus has shifted towards factor Xa inhibitors, or even dual thrombin and fXa inhibitors that have a broader mechanism of action by both inhibiting factor IIa (thrombin) and Xa. A recent review of patents and literature on thrombin inhibitors has demonstrated that the development of allosteric and multi-mechanism inhibitors might lead the way to a safer anticoagulant.