Related Research Articles
Hydrolysis is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Galactose, sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epimer of glucose. A galactose molecule linked with a glucose molecule forms a lactose molecule.

Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism.

Maltase is one type of alpha-glucosidase enzymes located in the brush border of the small intestine. This enzyme catalyzes the hydrolysis of disaccharide maltose into two simple sugars of glucose. Maltase is found in plants, bacteria, yeast, humans, and other vertebrates. It is thought to be synthesized by cells of the mucous membrane lining the intestinal wall.
An oligosaccharide is a saccharide polymer containing a small number of monosaccharides. Oligosaccharides can have many functions including cell recognition and cell adhesion.
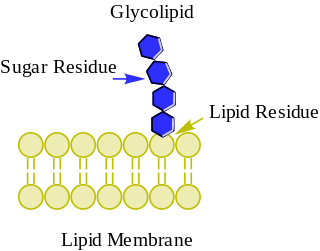
Glycolipids are lipids with a carbohydrate attached by a glycosidic (covalent) bond. Their role is to maintain the stability of the cell membrane and to facilitate cellular recognition, which is crucial to the immune response and in the connections that allow cells to connect to one another to form tissues. Glycolipids are found on the surface of all eukaryotic cell membranes, where they extend from the phospholipid bilayer into the extracellular environment.

Acarbose (INN) is an anti-diabetic drug used to treat diabetes mellitus type 2 and, in some countries, prediabetes. It is a generic sold in Europe and China as Glucobay, in North America as Precose, and in Canada as Prandase. It is cheap and popular in China, but not in the U.S. One physician explains that use in the U.S. is limited because it is not potent enough to justify the side effects of diarrhea and flatulence. However, a large study concluded in 2013 that "acarbose is effective, safe and well tolerated in a large cohort of Asian patients with type 2 diabetes." A possible explanation for the differing opinions is an observation that acarbose is significantly more effective in patients eating a relatively high carbohydrate Eastern diet.
PEP group translocation, also known as the phosphotransferase system or PTS, is a distinct method used by bacteria for sugar uptake where the source of energy is from phosphoenolpyruvate (PEP). It is known to be a multicomponent system that always involves enzymes of the plasma membrane and those in the cytoplasm.

The enzyme Trehalase is a glycoside hydrolase, produced by cells in the brush border of the small intestine, which catalyzes the conversion of trehalose to glucose. It is found in most animals.
Glycogenin is an enzyme involved in converting glucose to glycogen. It acts as a primer, by polymerizing the first few glucose molecules, after which other enzymes take over. It is a homodimer of 37-kDa subunits and is classified as a glycosyltransferase.
The terms glycans and polysaccharides are defined by IUPAC as synonyms meaning "compounds consisting of a large number of monosaccharides linked glycosidically". However, in practice the term glycan may also be used to refer to the carbohydrate portion of a glycoconjugate, such as a glycoprotein, glycolipid, or a proteoglycan, even if the carbohydrate is only an oligosaccharide. Glycans usually consist solely of O-glycosidic linkages of monosaccharides. For example, cellulose is a glycan composed of β-1,4-linked D-glucose, and chitin is a glycan composed of β-1,4-linked N-acetyl-D-glucosamine. Glycans can be homo- or heteropolymers of monosaccharide residues, and can be linear or branched.
In enzymology, a polysaccharide O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a quinoprotein glucose dehydrogenase is an enzyme that catalyzes the chemical reaction

The UDP-forming form of cellulose synthase is the main enzyme that produces cellulose. Systematically, it is known as UDP-glucose:(1→4)-β-D-glucan 4-β-D-glucosyltransferase in enzymology. It catalyzes the chemical reaction:
In enzymology, a dolichyl-phosphate beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphatidylglycerol-membrane-oligosaccharide glycerophosphotransferase is an enzyme that catalyzes the chemical reaction
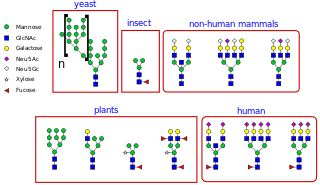
N-linked glycosylation, is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom, in a process called N-glycosylation, studied in biochemistry. The resulting protein is called an N-linked glycan, or simply an N-glycan.
Soluble quinoprotein glucose dehydrogenase is an enzyme with systematic name D-glucose:acceptor oxidoreductase. This enzyme catalyses the following chemical reaction

Alpha-1,2-glucosyltransferase ALG10-A is an enzyme that in humans is encoded by the ALG10 gene. It is a member of the Dolichyl-P-Glc:Glc2Man9GlcNAc2-PP-dolichol alpha-1,2-glucosyltransferase class of enzymes.
The enterobacterial common antigen (ECA) is a carbohydrate antigen found in the outer membrane of many Enterobacterales species. The antigen is unanimously absent from other gram-negative and gram-positive bacteria. Aeromonas hydrophila 209A is the only organism outside of Enterobacterales that expresses the ECA. More studies are needed to explain the presence of the antigen in this species as no other strains of this species express the antigen. The ECA is a polysaccharide made of repeating units of trisaccharides. The functions of these units have very few proven functions. Some evidence indicates role in pathogenicity in the bacteria that present the ECA. There are three separate types of ECA these include ECAPG, ECALPS, and ECACYC, each have different lengths. The synthesis of the ECA is controlled by the wec operon and has a 12-step synthesis which is described below. Due to the lack of proven function of the ECA, any clinical significance is hard to define however, some evidence suggests that human serum has antibodies against ECA.
References
- Goldberg DE, Rumley MK, Kennedy EP (1981). "Biosynthesis of membrane-derived oligosaccharides: a periplasmic phosphoglyceroltransferase". Proc. Natl. Acad. Sci. U.S.A. 78 (9): 5513–7. Bibcode:1981PNAS...78.5513G. doi: 10.1073/pnas.78.9.5513 . PMC 348776 . PMID 6272307.