
Nuclear fusion is a reaction in which two or more atomic nuclei, combine to form one or more atomic nuclei and neutrons. The difference in mass between the reactants and products is manifested as either the release or absorption of energy. This difference in mass arises as a result of the difference in nuclear binding energy between the atomic nuclei before and after the fusion reaction. Nuclear fusion is the process that powers active or main-sequence stars and other high-magnitude stars, where large amounts of energy are released.
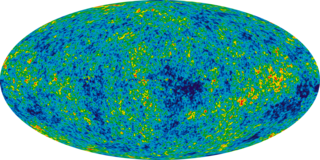
In physical cosmology, Big Bang nucleosynthesis is a model for the production of light nuclei, deuterium, 3He, 4He, 7Li, between 0.01s and 200s in the lifetime of the universe. The model uses a combination of thermodynamic arguments and results from equations for the expansion of the universe to define a changing temperature and density, then analyzes the rates of nuclear reactions at these temperatures and densities to predict the nuclear abundance ratios. Refined models agree very well with observations with the exception of the abundance of 7Li. The model is one of the key concepts in standard cosmology.

Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in a process called Big Bang nucleosynthesis. After about 20 minutes, the universe had expanded and cooled to a point at which these high-energy collisions among nucleons ended, so only the fastest and simplest reactions occurred, leaving our universe containing hydrogen and helium. The rest is traces of other elements such as lithium and the hydrogen isotope deuterium. Nucleosynthesis in stars and their explosions later produced the variety of elements and isotopes that we have today, in a process called cosmic chemical evolution. The amounts of total mass in elements heavier than hydrogen and helium remains small, so that the universe still has approximately the same composition.
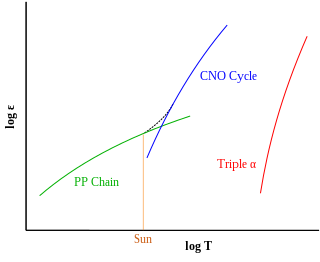
In astrophysics, stellar nucleosynthesis is the creation of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a predictive theory, it yields accurate estimates of the observed abundances of the elements. It explains why the observed abundances of elements change over time and why some elements and their isotopes are much more abundant than others. The theory was initially proposed by Fred Hoyle in 1946, who later refined it in 1954. Further advances were made, especially to nucleosynthesis by neutron capture of the elements heavier than iron, by Margaret and Geoffrey Burbidge, William Alfred Fowler and Fred Hoyle in their famous 1957 B2FH paper, which became one of the most heavily cited papers in astrophysics history.

The alpha process, also known as alpha capture or the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements. The other class is a cycle of reactions called the triple-alpha process, which consumes only helium, and produces carbon. The alpha process most commonly occurs in massive stars and during supernovae.
In astrophysics, silicon burning is a very brief sequence of nuclear fusion reactions that occur in massive stars with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the fuels that power them for their long lives in the main sequence on the Hertzsprung–Russell diagram. It follows the previous stages of hydrogen, helium, carbon, neon and oxygen burning processes.

In nuclear astrophysics, the rapid neutron-capture process, also known as the r-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", with the other half produced by the p-process and s-process. The r-process usually synthesizes the most neutron-rich stable isotopes of each heavy element. The r-process can typically synthesize the heaviest four isotopes of every heavy element; of these, the heavier two are called r-only nuclei because they are created exclusively via the r-process. Abundance peaks for the r-process occur near mass numbers A = 82, A = 130 and A = 196.

The slow neutron-capture process, or s-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The s-process is responsible for the creation (nucleosynthesis) of approximately half the atomic nuclei heavier than iron.

Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically.

The Oddo–Harkins rule holds that an element with an even atomic number is more abundant than the elements with immediately adjacent atomic numbers. For example, carbon, with atomic number 6, is more abundant than boron (5) and nitrogen (7). Generally, the relative abundance of an even atomic numbered element is roughly two orders of magnitude greater than the relative abundances of the immediately adjacent odd atomic numbered elements to either side. This pattern was first reported by Giuseppe Oddo in 1914 and William Draper Harkins in 1917. The Oddo–Harkins rule is true for all elements beginning with carbon produced by stellar nucleosynthesis but not true for the lightest elements below carbon produced by big bang nucleosynthesis and cosmic ray spallation.
Supernova nucleosynthesis is the nucleosynthesis of chemical elements in supernova explosions.
Cosmic ray spallation, also known as the x-process, is a set of naturally occurring nuclear reactions causing nucleosynthesis; it refers to the formation of chemical elements from the impact of cosmic rays on an object. Cosmic rays are highly energetic charged particles from beyond Earth, ranging from protons, alpha particles, and nuclei of many heavier elements. About 1% of cosmic rays also consist of free electrons.

Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is always a positive number, as the nucleus must gain energy for the nucleons to move apart from each other. Nucleons are attracted to each other by the strong nuclear force. In theoretical nuclear physics, the nuclear binding energy is considered a negative number. In this context it represents the energy of the nucleus relative to the energy of the constituent nucleons when they are infinitely far apart. Both the experimental and theoretical views are equivalent, with slightly different emphasis on what the binding energy means.

Nuclear astrophysics studies the origin of the chemical elements and isotopes, and the role of nuclear energy generation, in cosmic sources such as stars, supernovae, novae, and violent binary-star interactions. It is an interdisciplinary part of both nuclear physics and astrophysics, involving close collaboration among researchers in various subfields of each of these fields. This includes, notably, nuclear reactions and their rates as they occur in cosmic environments, and modeling of astrophysical objects where these nuclear reactions may occur, but also considerations of cosmic evolution of isotopic and elemental composition (often called chemical evolution). Constraints from observations involve multiple messengers, all across the electromagnetic spectrum (nuclear gamma-rays, X-rays, optical, and radio/sub-mm astronomy), as well as isotopic measurements of solar-system materials such as meteorites and their stardust inclusions, cosmic rays, material deposits on Earth and Moon). Nuclear physics experiments address stability (i.e., lifetimes and masses) for atomic nuclei well beyond the regime of stable nuclides into the realm of radioactive/unstable nuclei, almost to the limits of bound nuclei (the drip lines), and under high density (up to neutron star matter) and high temperature (plasma temperatures up to 109 K). Theories and simulations are essential parts herein, as cosmic nuclear reaction environments cannot be realized, but at best partially approximated by experiments.

Photodisintegration is a nuclear process in which an atomic nucleus absorbs a high-energy gamma ray, enters an excited state, and immediately decays by emitting a subatomic particle. The incoming gamma ray effectively knocks one or more neutrons, protons, or an alpha particle out of the nucleus. The reactions are called (γ,n), (γ,p), and (γ,α), respectively.
Nickel-62 is an isotope of nickel having 28 protons and 34 neutrons.
The B2FH paper was a landmark scientific paper on the origin of the chemical elements. The paper's title is Synthesis of the Elements in Stars, but it became known as B2FH from the initials of its authors: Margaret Burbidge, Geoffrey Burbidge, William A. Fowler, and Fred Hoyle. It was written from 1955 to 1956 at the University of Cambridge and Caltech, then published in Reviews of Modern Physics in 1957.
Neutron capture nucleosynthesis describes two nucleosynthesis pathways: the r-process and the s-process, for rapid and slow neutron captures, respectively. R-process describes neutron capture in a region of high neutron flux, such as during supernova nucleosynthesis after core-collapse, and yields neutron-rich nuclides. S-process describes neutron capture that is slow relative to the rate of beta decay, as for stellar nucleosynthesis in some stars, and yields nuclei with stable nuclear shells. Each process is responsible for roughly half of the observed abundances of elements heavier than iron. The importance of neutron capture to the observed abundance of the chemical elements was first described in 1957 in the B2FH paper.

The nuclear drip line is the boundary beyond which atomic nuclei are unbound with respect to the emission of a proton or neutron.
p-nuclei (p stands for proton-rich) are certain proton-rich, naturally occurring isotopes of some elements between selenium and mercury inclusive which cannot be produced in either the s- or the r-process.









