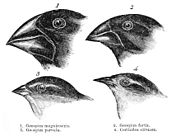
Speciation is the evolutionary process by which populations evolve to become distinct species. The biologist Orator F. Cook coined the term in 1906 for cladogenesis, the splitting of lineages, as opposed to anagenesis, phyletic evolution within lineages. Charles Darwin was the first to describe the role of natural selection in speciation in his 1859 book On the Origin of Species. He also identified sexual selection as a likely mechanism, but found it problematic.
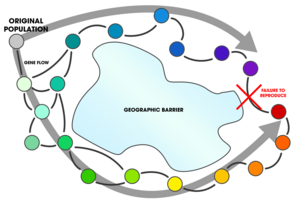
Allopatric speciation – also referred to as geographic speciation, vicariant speciation, or its earlier name the dumbbell model – is a mode of speciation that occurs when biological populations become geographically isolated from each other to an extent that prevents or interferes with gene flow.

In evolutionary biology, sympatric speciation is the evolution of a new species from a surviving ancestral species while both continue to inhabit the same geographic region. In evolutionary biology and biogeography, sympatric and sympatry are terms referring to organisms whose ranges overlap so that they occur together at least in some places. If these organisms are closely related, such a distribution may be the result of sympatric speciation. Etymologically, sympatry is derived from Greek συν (sun-) 'together' and πατρίς (patrís) 'fatherland'. The term was coined by Edward Bagnall Poulton in 1904, who explains the derivation.

Haldane's rule is an observation about the early stage of speciation, formulated in 1922 by the British evolutionary biologist J. B. S. Haldane, that states that if — in a species hybrid — only one sex is inviable or sterile, that sex is more likely to be the heterogametic sex. The heterogametic sex is the one with two different sex chromosomes; in therian mammals, for example, this is the male.
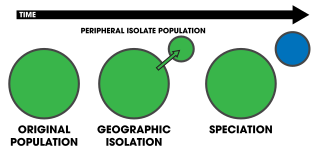
Peripatric speciation is a mode of speciation in which a new species is formed from an isolated peripheral population. Since peripatric speciation resembles allopatric speciation, in that populations are isolated and prevented from exchanging genes, it can often be difficult to distinguish between them., and peripatric speciation may be considered one type or model of allopatric speciation. The primary distinguishing characteristic of peripatric speciation is that one of the populations is much smaller than the other, as opposed to allopatric speciation, in which similarly-sized populations become separated. The terms peripatric and peripatry are often used in biogeography, referring to organisms whose ranges are closely adjacent but do not overlap, being separated where these organisms do not occur—for example on an oceanic island compared to the mainland. Such organisms are usually closely related ; their distribution being the result of peripatric speciation.

In biology, two related species or populations are considered sympatric when they exist in the same geographic area and thus frequently encounter one another. An initially interbreeding population that splits into two or more distinct species sharing a common range exemplifies sympatric speciation. Such speciation may be a product of reproductive isolation – which prevents hybrid offspring from being viable or able to reproduce, thereby reducing gene flow – that results in genetic divergence. Sympatric speciation may, but need not, arise through secondary contact, which refers to speciation or divergence in allopatry followed by range expansions leading to an area of sympatry. Sympatric species or taxa in secondary contact may or may not interbreed.

A hybrid zone exists where the ranges of two interbreeding species or diverged intraspecific lineages meet and cross-fertilize. Hybrid zones can form in situ due to the evolution of a new lineage but generally they result from secondary contact of the parental forms after a period of geographic isolation, which allowed their differentiation. Hybrid zones are useful in studying the genetics of speciation as they can provide natural examples of differentiation and gene flow between populations that are at some point on the continuum between diverging populations and separate species with reproductive isolation.
The mechanisms of reproductive isolation are a collection of evolutionary mechanisms, behaviors and physiological processes critical for speciation. They prevent members of different species from producing offspring, or ensure that any offspring are sterile. These barriers maintain the integrity of a species by reducing gene flow between related species.
In biology, a cline is a measurable gradient in a single characteristic of a species across its geographical range. Clines usually have a genetic, or phenotypic character. They can show either smooth, continuous gradation in a character, or more abrupt changes in the trait from one geographic region to the next.
H. Allen Orr is the Shirley Cox Kearns Professor of Biology at the University of Rochester.

The Bateson–Dobzhansky–Muller model, also known as Dobzhansky–Muller model, is a model of the evolution of genetic incompatibility, important in understanding the evolution of reproductive isolation during speciation and the role of natural selection in bringing it about. The theory was first described by William Bateson in 1909, then independently described by Theodosius Dobzhansky in 1934, and later elaborated in different forms by Herman Muller, H. Allen Orr and Sergey Gavrilets.

Ecological speciation is a form of speciation arising from reproductive isolation that occurs due to an ecological factor that reduces or eliminates gene flow between two populations of a species. Ecological factors can include changes in the environmental conditions in which a species experiences, such as behavioral changes involving predation, predator avoidance, pollinator attraction, and foraging; as well as changes in mate choice due to sexual selection or communication systems. Ecologically-driven reproductive isolation under divergent natural selection leads to the formation of new species. This has been documented in many cases in nature and has been a major focus of research on speciation for the past few decades.

Reinforcement is a process of speciation where natural selection increases the reproductive isolation between two populations of species. This occurs as a result of selection acting against the production of hybrid individuals of low fitness. The idea was originally developed by Alfred Russel Wallace and is sometimes referred to as the Wallace effect. The modern concept of reinforcement originates from Theodosius Dobzhansky. He envisioned a species separated allopatrically, where during secondary contact the two populations mate, producing hybrids with lower fitness. Natural selection results from the hybrid's inability to produce viable offspring; thus members of one species who do not mate with members of the other have greater reproductive success. This favors the evolution of greater prezygotic isolation. Reinforcement is one of the few cases in which selection can favor an increase in prezygotic isolation, influencing the process of speciation directly. This aspect has been particularly appealing among evolutionary biologists.

Secondary contact is the process in which two allopatrically distributed populations of a species are geographically reunited. This contact allows for the potential for the exchange of genes, dependent on how reproductively isolated the two populations have become. There are several primary outcomes of secondary contact: extinction of one species, fusion of the two populations back into one, reinforcement, the formation of a hybrid zone, and the formation of a new species through hybrid speciation.

The scientific study of speciation — how species evolve to become new species — began around the time of Charles Darwin in the middle of the 19th century. Many naturalists at the time recognized the relationship between biogeography and the evolution of species. The 20th century saw the growth of the field of speciation, with major contributors such as Ernst Mayr researching and documenting species' geographic patterns and relationships. The field grew in prominence with the modern evolutionary synthesis in the early part of that century. Since then, research on speciation has expanded immensely.

Reinforcement is a process within speciation where natural selection increases the reproductive isolation between two populations of species by reducing the production of hybrids. Evidence for speciation by reinforcement has been gathered since the 1990s, and along with data from comparative studies and laboratory experiments, has overcome many of the objections to the theory. Differences in behavior or biology that inhibit formation of hybrid zygotes are termed prezygotic isolation. Reinforcement can be shown to be occurring by measuring the strength of prezygotic isolation in a sympatric population in comparison to an allopatric population of the same species. Comparative studies of this allow for determining large-scale patterns in nature across various taxa. Mating patterns in hybrid zones can also be used to detect reinforcement. Reproductive character displacement is seen as a result of reinforcement, so many of the cases in nature express this pattern in sympatry. Reinforcement's prevalence is unknown, but the patterns of reproductive character displacement are found across numerous taxa, and is considered to be a common occurrence in nature. Studies of reinforcement in nature often prove difficult, as alternative explanations for the detected patterns can be asserted. Nevertheless, empirical evidence exists for reinforcement occurring across various taxa and its role in precipitating speciation is conclusive.

Laboratory experiments of speciation have been conducted for all four modes of speciation: allopatric, peripatric, parapatric, and sympatric; and various other processes involving speciation: hybridization, reinforcement, founder effects, among others. Most of the experiments have been done on flies, in particular Drosophila fruit flies. However, more recent studies have tested yeasts, fungi, and even viruses.
This glossary of genetics and evolutionary biology is a list of definitions of terms and concepts used in the study of genetics and evolutionary biology, as well as sub-disciplines and related fields, with an emphasis on classical genetics, quantitative genetics, population biology, phylogenetics, speciation, and systematics. It has been designed as a companion to Glossary of cellular and molecular biology, which contains many overlapping and related terms; other related glossaries include Glossary of biology and Glossary of ecology.
Allochronic speciation is a form of speciation arising from reproductive isolation that occurs due to a change in breeding time that reduces or eliminates gene flow between two populations of a species. The term allochrony is used to describe the general ecological phenomenon of the differences in phenology that arise between two or more species—speciation caused by allochrony is effectively allochronic speciation.
In biology, parallel speciation is a type of speciation where there is repeated evolution of reproductively isolating traits via the same mechanisms occurring between separate yet closely related species inhabiting different environments. This leads to a circumstance where independently evolved lineages have developed reproductive isolation from their ancestral lineage, but not from other independent lineages that inhabit similar environments. In order for parallel speciation to be confirmed, there is a set of three requirements that has been established that must be met: there must be phylogenetic independence between the separate populations inhabiting similar environments to ensure that the traits responsible for reproductive isolation evolved separately, there must be reproductive isolation not only between the ancestral population and the descendent population, but also between descendent populations that inhabit dissimilar environments, and descendent populations that inhabit similar environments must not be reproductively isolated from one another. To determine if natural selection specifically is the cause of parallel speciation, a fourth requirement has been established that includes identifying and testing an adaptive mechanism, which eliminates the possibility of a genetic factor such as polyploidy being the responsible agent.