
Glycolysis is the metabolic pathway that converts glucose into pyruvate, and in most organisms, occurs in the liquid part of cells, the cytosol. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

In biochemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology. Protein phosphorylation often activates many enzymes.
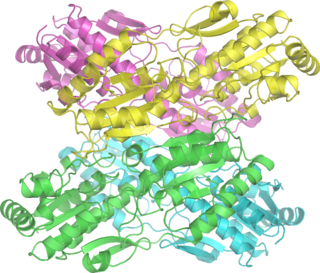
Phosphofructokinase-1 (PFK-1) is one of the most important regulatory enzymes of glycolysis. It is an allosteric enzyme made of 4 subunits and controlled by many activators and inhibitors. PFK-1 catalyzes the important "committed" step of glycolysis, the conversion of fructose 6-phosphate and ATP to fructose 1,6-bisphosphate and ADP. Glycolysis is the foundation for respiration, both anaerobic and aerobic. Because phosphofructokinase (PFK) catalyzes the ATP-dependent phosphorylation to convert fructose-6-phosphate into fructose 1,6-bisphosphate and ADP, it is one of the key regulatory steps of glycolysis. PFK is able to regulate glycolysis through allosteric inhibition, and in this way, the cell can increase or decrease the rate of glycolysis in response to the cell's energy requirements. For example, a high ratio of ATP to ADP will inhibit PFK and glycolysis. The key difference between the regulation of PFK in eukaryotes and prokaryotes is that in eukaryotes PFK is activated by fructose 2,6-bisphosphate. The purpose of fructose 2,6-bisphosphate is to supersede ATP inhibition, thus allowing eukaryotes to have greater sensitivity to regulation by hormones like glucagon and insulin.

Diphosphate—fructose-6-phosphate 1-phosphotransferase also known as PFP is an enzyme of carbohydrate metabolism in plants and some bacteria. The enzyme catalyses the reversible interconversion of fructose 6-phosphate and fructose 1,6-bisphosphate using inorganic pyrophosphate as the phosphoryl donor:

Phosphoglucomutase is an enzyme that transfers a phosphate group on an α-D-glucose monomer from the 1 to the 6 position in the forward direction or the 6 to the 1 position in the reverse direction.
A futile cycle, also known as a substrate cycle, occurs when two metabolic pathways run simultaneously in opposite directions and have no overall effect other than to dissipate energy in the form of heat. The reason this cycle was called "futile" cycle was because it appeared that this cycle operated with no net utility for the organism. As such, it was thought of being a quirk of the metabolism and thus named a futile cycle. After further investigation it was seen that futile cycles are very important for regulating the concentrations of metabolites. For example, if glycolysis and gluconeogenesis were to be active at the same time, glucose would be converted to pyruvate by glycolysis and then converted back to glucose by gluconeogenesis, with an overall consumption of ATP. Futile cycles may have a role in metabolic regulation, where a futile cycle would be a system oscillating between two states and very sensitive to small changes in the activity of any of the enzymes involved. The cycle does generate heat, and may be used to maintain thermal homeostasis, for example in the brown adipose tissue of young mammals, or to generate heat rapidly, for example in insect flight muscles and in hibernating animals during periodical arousal from torpor. It has been reported that the glucose metabolism substrate cycle is not a futile cycle but a regulatory process. For example, when energy is suddenly needed, ATP is replaced by AMP, a much more reactive adenine.

Fructose-bisphosphate aldolase, often just aldolase, is an enzyme catalyzing a reversible reaction that splits the aldol, fructose 1,6-bisphosphate, into the triose phosphates dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P). Aldolase can also produce DHAP from other (3S,4R)-ketose 1-phosphates such as fructose 1-phosphate and sedoheptulose 1,7-bisphosphate. Gluconeogenesis and the Calvin cycle, which are anabolic pathways, use the reverse reaction. Glycolysis, a catabolic pathway, uses the forward reaction. Aldolase is divided into two classes by mechanism.
Glucose-1,6-bisphosphate synthase is a type of enzyme called a phosphotransferase and is involved in mammalian starch and sucrose metabolism. It catalyzes the transfer of a phosphate group from 1,3-bisphosphoglycerate to glucose-1-phosphate, yielding 3-phosphoglycerate and glucose-1,6-bisphosphate.
In enzymology, a phosphoglucomutase (glucose-cofactor) is an enzyme that catalyzes the chemical reaction
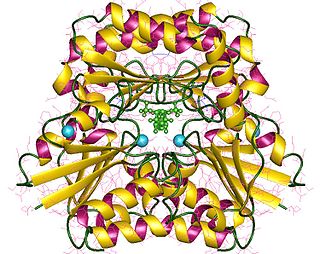
In enzymology, a phosphomannomutase is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphopentomutase is an enzyme that catalyzes the chemical reaction
In enzymology, a 1-phosphatidylinositol-5-phosphate 4-kinase is an enzyme that catalyzes the chemical reaction

In enzymology, 1-phosphofructokinase is an enzyme that catalyzes the chemical reaction
In enzymology, an ADP-specific phosphofructokinase is an enzyme that catalyzes the chemical reaction
In enzymology, a dihydrostreptomycin-6-phosphate 3'alpha-kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a glucose-1-phosphate phosphodismutase is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphoribokinase is an enzyme that catalyzes the chemical reaction
In enzymology, a ribose 1,5-bisphosphate phosphokinase is an enzyme that catalyzes the chemical reaction
In enzymology, a tagatose-6-phosphate kinase is an enzyme that catalyzes the chemical reaction
Bisphosphate may refer to:







