
Malaria is a mosquito-borne infectious disease that affects humans and other vertebrates. Human malaria causes symptoms that typically include fever, fatigue, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. Symptoms usually begin 10 to 15 days after being bitten by an infected Anopheles mosquito. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria.

Plasmodium is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of Plasmodium species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in malaria. During this infection, some parasites are picked up by a blood-feeding insect, continuing the life cycle.

Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. It is responsible for around 50% of all malaria cases. P. falciparum is therefore regarded as the deadliest parasite in humans. It is also associated with the development of blood cancer and is classified as a Group 2A (probable) carcinogen.

A gametocyte is a eukaryotic germ cell that divides by mitosis into other gametocytes or by meiosis into gametids during gametogenesis. Male gametocytes are called spermatocytes, and female gametocytes are called oocytes.
Recrudescence is the recurrence of an undesirable condition. In medicine, it is usually defined as the recurrence of symptoms after a period of remission or quiescence, in which sense it can sometimes be synonymous with relapse. In a narrower sense, it can also be such a recurrence with higher severity than before the remission. "Relapse" conventionally has a specific meaning when used in relation to malaria.

Plasmodium vivax is a protozoal parasite and a human pathogen. This parasite is the most frequent and widely distributed cause of recurring malaria. Although it is less virulent than Plasmodium falciparum, the deadliest of the five human malaria parasites, P. vivax malaria infections can lead to severe disease and death, often due to splenomegaly. P. vivax is carried by the female Anopheles mosquito; the males do not bite.

Plasmodium ovale is a species of parasitic protozoon that causes tertian malaria in humans. It is one of several species of Plasmodium parasites that infect humans, including Plasmodium falciparum and Plasmodium vivax which are responsible for most cases of malaria in the world. P. ovale is rare compared to these two parasites, and substantially less dangerous than P. falciparum.

Plasmodium malariae is a parasitic protozoan that causes malaria in humans. It is one of several species of Plasmodium parasites that infect other organisms as pathogens, also including Plasmodium falciparum and Plasmodium vivax, responsible for most malarial infection. Found worldwide, it causes a so-called "benign malaria", not nearly as dangerous as that produced by P. falciparum or P. vivax. The signs include fevers that recur at approximately three-day intervals – a quartan fever or quartan malaria – longer than the two-day (tertian) intervals of the other malarial parasite.

Plasmodium knowlesi is a parasite that causes malaria in humans and other primates. It is found throughout Southeast Asia, and is the most common cause of human malaria in Malaysia. Like other Plasmodium species, P. knowlesi has a life cycle that requires infection of both a mosquito and a warm-blooded host. While the natural warm-blooded hosts of P. knowlesi are likely various Old World monkeys, humans can be infected by P. knowlesi if they are fed upon by infected mosquitoes. P. knowlesi is a eukaryote in the phylum Apicomplexa, genus Plasmodium, and subgenus Plasmodium. It is most closely related to the human parasite Plasmodium vivax as well as other Plasmodium species that infect non-human primates.
Plasmodium yoelii is a parasite of the genus Plasmodium subgenus Vinckeia. As in all Plasmodium species, P. yoelii has both vertebrate and insect hosts. The vertebrate hosts for this parasite are mammals.
Malaria vaccines are vaccines that prevent malaria, a mosquito-borne infectious disease which annually affects an estimated 247 million people worldwide and causes 619,000 deaths. The first approved vaccine for malaria is RTS,S, known by the brand name Mosquirix. As of April 2023, the vaccine has been given to 1.5 million children living in areas with moderate-to-high malaria transmission. It requires at least three doses in infants by age 2, and a fourth dose extends the protection for another 1–2 years. The vaccine reduces hospital admissions from severe malaria by around 30%.

The history of malaria extends from its prehistoric origin as a zoonotic disease in the primates of Africa through to the 21st century. A widespread and potentially lethal human infectious disease, at its peak malaria infested every continent except Antarctica. Its prevention and treatment have been targeted in science and medicine for hundreds of years. Since the discovery of the Plasmodium parasites which cause it, research attention has focused on their biology as well as that of the mosquitoes which transmit the parasites.
Hematozoa is a subclass of blood parasites of the Apicomplexa clade. Well known examples include the Plasmodium spp. which cause malaria in humans and Theileria which causes theileriosis in cattle. A large number of species are known to infect birds and are transmitted by insect vectors. The pattern in which Haematozoa infect a host cell depends on the genera of the blood parasite. Plasmodium and Leucozytozoon displace the nucleus of the host cell so that the parasite can take control of the cell where as Hemoproteus completely envelops the nucleus in a host cell.
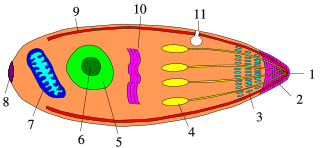
Apicomplexans, a group of intracellular parasites, have life cycle stages that allow them to survive the wide variety of environments they are exposed to during their complex life cycle. Each stage in the life cycle of an apicomplexan organism is typified by a cellular variety with a distinct morphology and biochemistry.
Plasmodium inui is a species of parasite, one of the species of simian Plasmodium that cause malaria in Old World monkeys.
Plasmodium coatneyi is a parasitic species that is an agent of malaria in nonhuman primates. P. coatneyi occurs in Southeast Asia. The natural host of this species is the rhesus macaque and crab-eating macaque, but there has been no evidence that zoonosis of P. coatneyi can occur through its vector, the female Anopheles mosquito.

Quartan fever is one of the four types of malaria which can be contracted by humans.
Mary R. Galinski is a professor of medicine at the Emory Vaccine Center, Hubert Department of Global Health of the Rollins School of Public Health, and the Department of Medicine of the Emory University School of Medicine.











