
Heteropodatoxins are peptide toxins from the venom of the giant crab spider Heteropoda venatoria, which block Kv4.2 voltage-gated potassium channels.
Stromatoxin is a spider toxin that blocks certain delayed-rectifier and A-type voltage-gated potassium channels.

Acid-sensing ion channel 1 (ASIC1) also known as amiloride-sensitive cation channel 2, neuronal (ACCN2) or brain sodium channel 2 (BNaC2) is a protein that in humans is encoded by the ASIC1 gene. The ASIC1 gene is one of the five paralogous genes that encode proteins that form trimeric acid-sensing ion channels (ASICs) in mammals. The cDNA of this gene was first cloned in 1996. The ASIC genes have splicing variants that encode different proteins that are called isoforms.

Acid-sensing ion channel 3 (ASIC3) also known as amiloride-sensitive cation channel 3 (ACCN3) or testis sodium channel 1 (TNaC1) is a protein that in humans is encoded by the ASIC3 gene. The ASIC3 gene is one of the five paralogous genes that encode proteins that form trimeric acid-sensing ion channels (ASICs) in mammals. The cDNA of this gene was first cloned in 1998. The ASIC genes have splicing variants that encode different proteins that are called isoforms.

Acid-sensing ion channel 2 (ASIC2) also known as amiloride-sensitive cation channel 1, neuronal (ACCN1) or brain sodium channel 1 (BNaC1) is a protein that in humans is encoded by the ASIC2 gene. The ASIC2 gene is one of the five paralogous genes that encode proteins that form trimeric acid-sensing ion channels (ASICs) in mammals. The cDNA of this gene was first cloned in 1996. The ASIC genes have splicing variants that encode different proteins that are called isoforms.
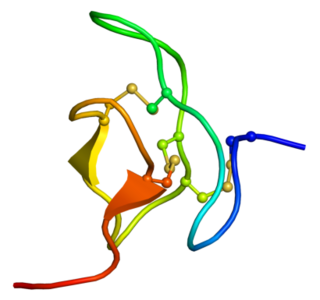
Phrixotoxins are peptide toxins derived from the venom of the Chilean copper tarantula Phrixotrichus auratus, also named Paraphysa scrofa. Phrixotoxin-1 and -2 block A-type voltage-gated potassium channels; phrixotoxin-3 blocks voltage-gated sodium channels. Similar toxins are found in other species, for instance the Chilean rose tarantula.

Acid-sensing ion channels (ASICs) are neuronal voltage-insensitive sodium channels activated by extracellular protons permeable to Na+. ASIC1 also shows low Ca2+ permeability. ASIC proteins are a subfamily of the ENaC/Deg superfamily of ion channels. These genes have splice variants that encode for several isoforms that are marked by a suffix. In mammals, acid-sensing ion channels (ASIC) are encoded by five genes that produce ASIC protein subunits: ASIC1, ASIC2, ASIC3, ASIC4, and ASIC5. Three of these protein subunits assemble to form the ASIC, which can combine into both homotrimeric and heterotrimeric channels typically found in both the central nervous system and peripheral nervous system. However, the most common ASICs are ASIC1a and ASIC1a/2a and ASIC3. ASIC2b is non-functional on its own but modulates channel activity when participating in heteromultimers and ASIC4 has no known function. On a broad scale, ASICs are potential drug targets due to their involvement in pathological states such as retinal damage, seizures, and ischemic brain injury.

Heteroscodratoxin-1 is a neurotoxin produced by the venom glands of Heteroscodra maculata that shifts the activation threshold of voltage-gated potassium channels and the inactivation of Nav1.1 sodium channels to more positive potentials.

Vanillotoxins are neurotoxins found in the venom of the tarantula Psalmopoeus cambridgei. They act as agonists for the transient receptor potential cation channel subfamily V member 1 (TRPV1), activating the pain sensory system. VaTx1 and 2 also act as antagonists for the Kv2-type voltage-gated potassium channel (Kv2), inducing paralytic behavior in small animals.

Guangxitoxin, also known as GxTX, is a peptide toxin found in the venom of the tarantula Plesiophrictus guangxiensis. It primarily inhibits outward voltage-gated Kv2.1 potassium channel currents, which are prominently expressed in pancreatic β-cells, thus increasing insulin secretion.
Hanatoxin is a toxin found in the venom of the Grammostola spatulata tarantula. The toxin is mostly known for inhibiting the activation of voltage-gated potassium channels, most specifically Kv4.2 and Kv2.1, by raising its activation threshold.

Mambalgins are peptides found in the venom of the black mamba, an elapid snake. Mambalgins are members of the three-finger toxin (3FTx) protein family and have the characteristic three-finger protein fold. First reported by French researchers in 2012, mambalgins are unusual members of the 3FTx family in that they have the in vivo effect of causing analgesia without apparent toxicity. Their mechanism of action is potent inhibition of acid-sensing ion channels.
HsTx1 is a toxin from the venom of the scorpion Heterometrus spinifer. HsTx1 is a very potent inhibitor of the rat Kv1.3 voltage-gated potassium channel.
μ-THTX-Cl6a, also known as Cl6a, is a 33-residue peptide toxin extracted from the venom of the spider Cyriopagopus longipes. The toxin acts as an inhibitor of the tetrodotoxin-sensitive (TTX-S) voltage-gated sodium channel (NaV1.7), thereby causing sustained reduction of NaV1.7 currents.
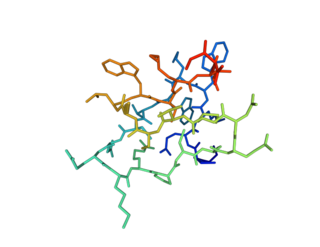
GiTx1 (β/κ-theraphotoxin-Gi1a) is a peptide toxin present in the venom of Grammostola iheringi. It reduces both inward and outward currents by blocking voltage-gated sodium and potassium channels, respectively.
Protoxin-I, also known as ProTx-I, or Beta/omega-theraphotoxin-Tp1a, is a 35-amino-acid peptide neurotoxin extracted from the venom of the tarantula Thrixopelma pruriens. Protoxin-I belongs to the inhibitory cystine knot (ICK) family of peptide toxins, which have been known to potently inhibit voltage-gated ion channels. Protoxin-I selectively blocks low voltage threshold T-type calcium channels, voltage gated sodium channels and the nociceptor cation channel TRPA1. Due to its unique ability to bind to TRPA1, Protoxin-I has been implicated as a valuable pharmacological reagent with potential applications in clinical contexts with regards to pain and inflammation

Delta hexatoxin Hv1 is a neurotoxic component found in the venom of the Australian funnel web spider.

Double-knot toxin (DkTx), also known as Tau-theraphotoxin-Hs1a or Tau-TRTX-Hs1a, is a toxin found in the venom of the Chinese Bird spider, a tarantula species primarily living in the Guangxi province of China. This toxin, characterized by its bivalent structure of two Inhibitor Cysteine Knots (ICK), is thought to induce excruciating and long-lasting pain by activating the transient receptor potential vanilloid 1 (TRPV1) channel.

Grammostola mechanotoxin #4, also known as M-theraphotoxin-Gr1a (M-TRTX-Gr1a), is a neurotoxin isolated from the venom of the spider Chilean rose tarantula Grammostola spatulate. This amphiphilic peptide, which consists of 35 amino acids, belongs to the inhibitory cysteine knot (ICK) peptide family. It reduces mechanical sensation by inhibiting mechanosensitive channels (MSCs).
Phlotoxin is a neurotoxin from the venom of the tarantula Phlogiellus that targets mostly voltage-sensitive sodium channels and mainly Nav1.7. The only non-sodium voltage-sensitive channel that is inhibited by Phlotoxin is Kv3.4. Nav1.4 and Nav1.6 seem to be Phlotoxin-1-sensitive to some extent as well.















