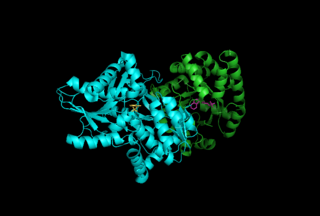
Tryptophan synthase or tryptophan synthetase is an enzyme that catalyses the final two steps in the biosynthesis of tryptophan. It is commonly found in Eubacteria, Archaebacteria, Protista, Fungi, and Plantae. However, it is absent from Animalia. It is typically found as an α2β2 tetramer. The α subunits catalyze the reversible formation of indole and glyceraldehyde-3-phosphate (G3P) from indole-3-glycerol phosphate (IGP). The β subunits catalyze the irreversible condensation of indole and serine to form tryptophan in a pyridoxal phosphate (PLP) dependent reaction. Each α active site is connected to a β active site by a 25 angstrom long hydrophobic channel contained within the enzyme. This facilitates the diffusion of indole formed at α active sites directly to β active sites in a process known as substrate channeling. The active sites of tryptophan synthase are allosterically coupled.
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).
In enzymology, a phosphoethanolamine N-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an enterobactin synthase is an enzyme that catalyzes the chemical reaction
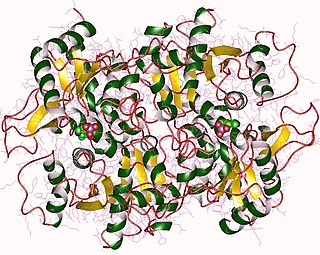
The enzyme threonine synthase (EC 4.2.3.1) catalyzes the chemical reaction
The enzyme serine-ethanolaminephosphate phosphodiesterase (EC 3.1.4.13) catalyzes the reaction
In enzymology, a cysteine synthase is an enzyme that catalyzes the chemical reaction
In enzymology, a CDP-diacylglycerol—glycerol-3-phosphate 3-phosphatidyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a CDP-diacylglycerol—inositol 3-phosphatidyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a CDP-diacylglycerol—serine O-phosphatidyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a CDP-glycerol glycerophosphotransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a CDP-ribitol ribitolphosphotransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an ethanolamine-phosphate cytidylyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an ethanolaminephosphotransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a N-acylneuraminate cytidylyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphatidylcholine synthase is an enzyme that catalyzes the chemical reaction
CDP-archaeol synthase is an enzyme with systematic name CTP:2,3-bis-O-(geranylgeranyl)-sn-glycero-1-phosphate cytidylyltransferase. This enzyme catalyses the following chemical reaction
In enzymology, a ceramide phosphoethanolamine synthase is an enzyme that catalyzes the chemical reaction





