
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenate (vitamin B5), and adenosine triphosphate (ATP).
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. It is produced by many plants and animals (including humans) and its polymers are the main component of natural rubber. C. G. Williams named the compound in 1860 after obtaining it from the pyrolysis of natural rubber; he correctly deduced the empirical formula C5H8.

Coenzyme Q is a coenzyme family that is ubiquitous in animals and most bacteria (hence its other name, ubiquinone). In humans, the most common form is coenzyme Q10 (which is also called CoQ10 and ubiquinone-10.
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists.

Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine.

Secondary metabolites, also called specialised metabolites, toxins, secondary products, or natural products, are organic compounds produced by any lifeform, e.g. bacteria, fungi, animals, or plants, which are not directly involved in the normal growth, development, or reproduction of the organism. Instead, they generally mediate ecological interactions, which may produce a selective advantage for the organism by increasing its survivability or fecundity. Specific secondary metabolites are often restricted to a narrow set of species within a phylogenetic group. Secondary metabolites often play an important role in plant defense against herbivory and other interspecies defenses. Humans use secondary metabolites as medicines, flavourings, pigments, and recreational drugs.

Phytochemistry is the study of phytochemicals, which are chemicals derived from plants. Phytochemists strive to describe the structures of the large number of secondary metabolites found in plants, the functions of these compounds in human and plant biology, and the biosynthesis of these compounds. Plants synthesize phytochemicals for many reasons, including to protect themselves against insect attacks and plant diseases. The compounds found in plants are of many kinds, but most can be grouped into four major biosynthetic classes: alkaloids, phenylpropanoids, polyketides, and terpenoids.

Noscapine is a benzylisoquinoline alkaloid, of the phthalideisoquinoline structural subgroup, which has been isolated from numerous species of the family Papaveraceae. It lacks significant hypnotic, euphoric, or analgesic effects affording it with very low addictive potential. This agent is primarily used for its antitussive (cough-suppressing) effects.

Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids. A recent study conducted in the Cosmics Leaving Outdoor Droplets large cloud chamber at CERN, has identified sesquiterpenes — gaseous hydrocarbons that are released by plants — as potentially playing a major role in cloud formation in relatively pristine regions of the atmosphere.
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP.
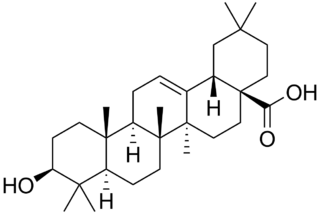
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins.

Capsidiol is a terpenoid compound that accumulates in tobacco Nicotiana tabacum and chili pepper Capsicum annuum in response to fungal infection. Capsidiol is categorized under the broad term of phytoalexin, a class of low molecular weight plant secondary metabolites that are produced during infection. Phytoalexins are also characterized as a part of a two pronged response to infection which involves a short term response consisting of production of free radicals near the site of infection and a long term response involving the production of hormones and an increase in enzymes to biosynthesize phytoalexins such as capsidiol.

Absinthin is a naturally produced triterpene lactone from the plant Artemisia absinthium (Wormwood). It constitutes one of the most bitter chemical agents responsible for absinthe's distinct taste. The compound shows biological activity and has shown promise as an anti-inflammatory agent, and should not be confused with thujone, a neurotoxin also found in Artemisia absinthium.

Xanthohumol is a natural product found in the female inflorescences of Humulus lupulus, also known as hops. This compound is also found in beer and belongs to a class of compounds that contribute to the bitterness and flavor of hops. Xanthohumol is a prenylated chalconoid, biosynthesized by a type III polyketide synthase (PKS) and subsequent modifying enzymes.
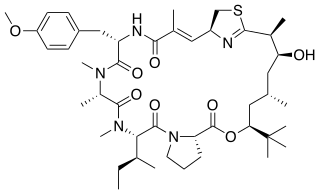
Apratoxin A - is a cyanobacterial secondary metabolite, known as a potent cytotoxic marine natural product. It is a derivative of the Apratoxin family of cytotoxins. The mixed peptide-polyketide natural product comes from a polyketide synthase/non-ribosomal peptide synthase pathway (PKS/NRPS). This cytotoxin is known for inducing G1-phase cell cycle arrest and apoptosis. This natural product's activity has made it a popular target for developing anticancer derivatives.

The biosynthesis of benzoxazinone, a cyclic hydroxamate and a natural insecticide, has been well-characterized in maize and related grass species. In maize, genes in the pathway are named using the symbol bx. Maize Bx-genes are tightly linked, a feature that has been considered uncommon for plant genes of a biosynthetic pathways. Especially notable are genes encoding the different enzymatic functions BX1, BX2 and BX8 and which are found within about 50 kilobases. Results from wheat and rye indicate that the cluster is an ancient feature. In wheat the cluster is split into two parts. The wheat genes Bx1 and Bx2 are located in close proximity on chromosome 4 and wheat Bx3, Bx4 and Bx5 map to the short arm of chromosome 5; an additional Bx3 copy was detected on the long arm of chromosome 5B. Recently, additional biosynthetic clusters have been detected in other plants for other biosynthetic pathways and this organization might be common in plants.

Arglabin is a sesquiterpene lactone belonging to the guaianolide subclass bearing a 5,7,5-tricyclic ring system which is known to inhibit farnesyl transferase. It is characterized by an epoxide on the cycloheptane as well as an exocyclic methylene group that is conjugated with the carbonyl of the lactone. Arglabin is extracted from Artemisia glabella, a species of wormwood, found in the Karaganda Region of Kazakhstan. Arglabin and its derivatives are biologically active and demonstrate promising antitumor activity and cytoxocity against varying tumor cell lines.
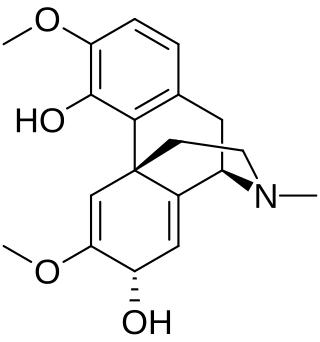
Salutaridinol is a modified benzyltetrahydroisoquinoline alkaloid with the formula C19H23NO4. It is produced in the secondary metabolism of the opium poppy Papaver somniferum (Papaveraceae) as an intermediate in the biosynthetic pathway that generates morphine. As an isoquinoline alkaloid, it is fundamentally derived from tyrosine as part of the shikimate pathway of secondary metabolism. Salutaridinol is a product of the enzyme salutaridine: NADPH 7-oxidoreductase and the substrate for the enzyme salutaridinol 7-O-acetyltransferase, which are two of the four enzymes in the morphine biosynthesis pathway that generates morphine from (R)-reticuline. Salutaridinol's unique position adjacent to two of the four enzymes in the morphine biosynthesis pathway gives it an important role in enzymatic, genetic, and synthetic biology studies of morphine biosynthesis. Salutaridinol levels are indicative of the flux through the morphine biosynthesis pathway and the efficacy of both salutaridine: NADPH 7-oxidoreductase and salutaridinol 7-O-acetyltransferase.

Cannabis (/ˈkænəbɪs/) is commonly known as marijuana or hemp and has two known strains: Cannabis sativa and Cannabis indica, both of which produce chemicals to deter herbivory. The chemical composition includes specialized terpenes and cannabinoids, mainly tetrahydrocannabinol (THC), and cannabidiol (CBD). These substances play a role in defending the plant from pathogens including insects, fungi, viruses and bacteria. THC and CBD are stored mostly in the trichomes of the plant, and can cause psychological and physical impairment in the user, via the endocannabinoid system and unique receptors. THC increases dopamine levels in the brain, which attributes to the euphoric and relaxed feelings cannabis provides. As THC is a secondary metabolite, it poses no known effects towards plant development, growth, and reproduction. However, some studies show secondary metabolites such as cannabinoids, flavonoids, and terpenes are used as defense mechanisms against biotic and abiotic environmental stressors.

Disorazol, a cyclic polyketide synthesized by the bacterium Sorangium cellulosum So ce12, was first detected and isolated in 1994. Its chemical structure consists of a macrocyclic ring and two oxazole rings. Disorazol A has been demonstrated to exhibit anti-fungi activities, but it was not active against yeasts. In addition, this substance demonstrates potent anti-cancer characteristics at exceptionally low picomolar levels by obstructing the mechanism of tubulin assembly and triggering the disruption of microtubules. As a result, these impacts lead to the initiation of cell apoptosis. However, disorazols cannot be directly used as drugs in the clinic due to its extremely high cytotoxicity and instability. Thus, chemical and biosynthetic synthesis pathways were designed to synthesize unnatural derivatives of disorazol in hope of reducing its cytotoxicity without decreasing its anti-cancer potency.

















