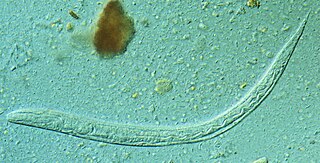
Strongyloides stercoralis is a human pathogenic parasitic roundworm causing the disease strongyloidiasis. Its common name in the US is threadworm. In the UK and Australia, however, the term threadworm can also refer to nematodes of the genus Enterobius, otherwise known as pinworms.

Hookworm infection is an infection by a type of intestinal parasite known as a hookworm. Initially, itching and a rash may occur at the site of infection. Those only affected by a few worms may show no symptoms. Those infected by many worms may experience abdominal pain, diarrhea, weight loss, and tiredness. The mental and physical development of children may be affected. Anemia may result.

Necator americanus is a species of hookworm commonly known as the New World hookworm. Like other hookworms, it is a member of the phylum Nematoda. It is an obligatory parasitic nematode that lives in the small intestine of human hosts. Necatoriasis—a type of helminthiasis—is the term for the condition of being host to an infestation of a species of Necator. Since N. americanus and Ancylostoma duodenale are the two species of hookworms that most commonly infest humans, they are usually dealt with under the collective heading of "hookworm infection". They differ most obviously in geographical distribution, structure of mouthparts, and relative size.

Ascaris suum, also known as the large roundworm of pig, is a parasitic nematode that causes ascariasis in pigs. While roundworms in pigs and humans are today considered as two species with different hosts, cross-infection between humans and pigs is possible; some researchers have thus argued they are the same species. Ascariasis is associated with contact to pigs and pig manure in Denmark.

Parasitic worms, also known as helminths, are a polyphyletic group of large macroparasites; adults can generally be seen with the naked eye. Many are intestinal worms that are soil-transmitted and infect the gastrointestinal tract. Other parasitic worms such as schistosomes reside in blood vessels.

Ancylostoma duodenale is a species of the roundworm genus Ancylostoma. It is a parasitic nematode worm and commonly known as the Old World hookworm. It lives in the small intestine especially the jejunum of definitive hosts, generally humans, where it is able to mate and mature. Ancylostoma duodenale and Necator americanus are the two human hookworm species that are normally discussed together as the cause of hookworm infection. They are dioecious. Ancylostoma duodenale is abundant throughout the world, including Southern Europe, North Africa, India, China, Southeast Asia, some areas in the United States, the Caribbean, and South America.
Uncinaria stenocephala is a nematode that parasitizes dogs, cats, and foxes as well as humans. It is rare to find in cats in the United States. Uncinaria stenocephala is the most common canine hookworm in cooler regions, such as Canada and the northern regions of the US, where it can be found primarily in foxes (40%). U. stenocephala is also one of the most common hookworms in the UK, called the northern hookworm, however it has a rather low prevalence. U. stenocephala is also considered to be zoonotic hookworms because they live in animals but can be transmitted to humans.

The Strongylida suborder includes many of the important nematodes found in the gastrointestinal tracts of ruminants, horses, and swine, as well as the lungworms of ruminants and the hookworms of dogs and cats.

Ancylostomiasis is a hookworm disease caused by infection with Ancylostoma hookworms. The name is derived from Greek ancylos αγκύλος "crooked, bent" and stoma στόμα "mouth".

Necatoriasis is the condition of infection by Necator hookworms, such as Necator americanus. This hookworm infection is a type of helminthiasis (infection) which is a type of neglected tropical disease.

Oesophagostomum is a genus of parasitic nematodes (roundworms) of the family Strongylidae. These worms occur in Africa, Brazil, China, Indonesia and the Philippines. The majority of human infection with Oesophagostomum is localized to northern Togo and Ghana. Because the eggs may be indistinguishable from those of the hookworms, the species causing human helminthomas are rarely identified with accuracy. Oesophagostomum, especially O. bifurcum, are common parasites of livestock and animals like goats, pigs and non-human primates, although it seems that humans are increasingly becoming favorable hosts as well. The disease they cause, oesophagostomiasis, is known for the nodule formation it causes in the intestines of its infected hosts, which can lead to more serious problems such as dysentery. Although the routes of human infection have yet to be elucidated sufficiently, it is believed that transmission occurs through oral-fecal means, with infected humans unknowingly ingesting soil containing the infectious filariform larvae.

Toxascaris leonina is a common parasitic roundworm found in dogs, cats, foxes, and related host species. T. leonina is an ascarid nematode, a worldwide distributed helminth parasite which is in a division of eukaryotic parasites that, unlike external parasites such as lice and fleas, live inside their host. The definitive hosts of T. leonina include canids and felines (cats), while the intermediate hosts are usually rodents, such as mice or rats. Infection occurs in the definitive host when the animal eats an infected rodent. While T. leonina can occur in either dogs or cats, it is far more frequent in cats.

Ancylostoma braziliense is a species of hookworm belonging to the genus Ancylostoma. It is an intestinal parasite of domestic cats and dogs. Severe infection is often fatal to these pets, especially in puppies and kittens. The infection is particularly endemic in the southern United States. It is most often confused with the zoonotic hookworm species Ancylostoma ceylanicum because of their uncanny resemblance.

The Ancylostomatidae are a family of worms that includes the hookworms.

Soil-transmitted helminthiasis is a type of worm infection (helminthiasis) caused by different species of roundworms. It is caused specifically by worms transmitted through soil contaminated with faecal matter and are known assoil-transmitted helminths. Three types of soil-transmitted helminthiasis can be distinguished: ascariasis, hookworm infection and whipworm infection. These three types of infection are therefore caused by the large roundworm A. lumbricoides, the hookworms Necator americanus or Ancylostoma duodenale and by the whipworm Trichuris trichiura.
Ancylostoma ceylanicum is a parasitic roundworm belonging to the genus Ancylostoma. It is a hookworm both of humans and of other mammals such as dogs, cats, and golden hamsters. It is the only zoonotic hookworm species that is able to produce symptomatic infections in humans, with the majority of cases being in Southeast Asia.

Cooperia oncophora is one of the most common intestinal parasitic nematodes in cattle in temperate regions. Infections with C. oncophora may result in mild clinical symptoms, but can lead to weight loss and damage of the small intestine, especially when co-infections with other nematodes such as O. ostertagi occur. Infections are usually treated with broad-spectrum anthelmintics such as benzimidazole, but resistance to these drugs has developed in the last decades and is now very common. C. oncophora has a direct life cycle. Infective larvae are ingested by the host. The larvae grow to adults, which reproduce in the small intestines. Eggs are shed onto the pasture with the faeces, which leads to new infections. Co-infections with other gastro-intestinal nematodes such as O. ostertagi and H. contortus are common.
Hookworms are intestinal, blood-feeding, parasitic roundworms that cause types of infection known as helminthiases. Hookworm infection is found in many parts of the world, and is common in areas with poor access to adequate water, sanitation, and hygiene. In humans, infections are caused by two main species of roundworm, belonging to the genera Ancylostoma and Necator. In other animals the main parasites are species of Ancylostoma. Hookworm is closely associated with poverty because it is most often found in impoverished areas, and its symptoms promote poverty through the educational and health effects it has on children. It is the leading cause of anemia and undernutrition in developing countries, while being one of the most commonly occurring diseases among poor people. Hookworm thrives in areas where rainfall is sufficient and keeps the soil from drying out, and where temperatures are higher, making rural, coastal areas prime conditions for the parasite to breed.

Cat worm infections, the infection of cats (Felidae) with parasitic worms, occur frequently. Most worm species occur worldwide in both domestic and other cats, but there are regional, species and lifestyle differences in the frequency of infestation. According to the classification of the corresponding parasites in the zoological system, infections can be divided into those caused by nematode and flatworms - in the case of the latter, mainly cestoda and trematoda - while other strains are of no veterinary significance. While threadworms usually do not require an intermediate host for their reproduction, the development cycle of flatworms always proceeds via alternate hosts.
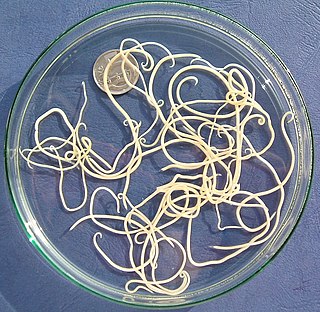
Nematode infection in dogs - the infection of dogs with parasitic nemamotodes - are, along with tapeworm infections and infections with protozoa, frequent parasitoses in veterinary practice. Nematodes, as so-called endoparasites, colonize various internal organs - most of them the digestive tract - and the skin. To date, about 30 different species of nematode have been identified in domestic dogs; they are essentially also found in wild dog species. However, the majority of them often cause no or only minor symptoms of disease in adult animals. The infection therefore does not necessarily have to manifest itself in a worm disease (helminthosis). For most nematodes, an infection can be detected by examining the feces for eggs or larvae. Roundworm infection in dogs and the hookworm in dogs is of particular health significance in Central Europe, as they can also be transmitted to humans (zoonosis). Regular deworming can significantly reduce the frequency of infection and thus the risk of infection for humans and dogs.



















