
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3), and is typically associated with the corrosion of refined iron.

In chemistry, iron(III) or ferric refers to the element iron in its +3 oxidation state. Ferric chloride is an alternative name for iron(III) chloride (FeCl3). The adjective ferrous is used instead for iron(II) salts, containing the cation Fe2+. The word ferric is derived from the Latin word ferrum, meaning "iron".
The pedosphere is the outermost layer of the Earth that is composed of soil and subject to soil formation processes. It exists at the interface of the lithosphere, atmosphere, hydrosphere and biosphere. The pedosphere is the skin of the Earth and only develops when there is a dynamic interaction between the atmosphere, biosphere, lithosphere and the hydrosphere. The pedosphere is the foundation of terrestrial life on Earth.

In soil science, podzols, also known as podosols, spodosols, or espodossolos, are the typical soils of coniferous or boreal forests and also the typical soils of eucalypt forests and heathlands in southern Australia. In Western Europe, podzols develop on heathland, which is often a construct of human interference through grazing and burning. In some British moorlands with podzolic soils, cambisols are preserved under Bronze Age barrows.
A soil horizon is a layer parallel to the soil surface whose physical, chemical and biological characteristics differ from the layers above and beneath. Horizons are defined in many cases by obvious physical features, mainly colour and texture. These may be described both in absolute terms and in terms relative to the surrounding material, i.e. 'coarser' or 'sandier' than the horizons above and below.

Entisols are soils, as defined under USDA soil taxonomy, that do not show any profile development other than an A-horizon. Entisols have no diagnostic horizons, and are unaltered from their parent material, which could be unconsolidated sediment, or rock. Entisols are the most common soils, occupying about 16% of the global ice-free land area.

The World Reference Base for Soil Resources (WRB) is an international soil classification system for naming soils and creating legends for soil maps. The currently valid version is the fourth edition 2022. It is edited by a working group of the International Union of Soil Sciences (IUSS).
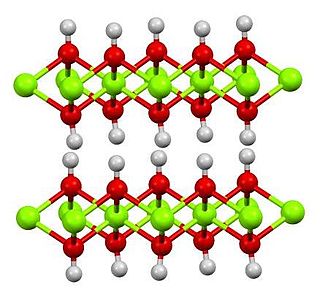
Iron (II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)2. It is produced when iron (II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but even traces of oxygen impart a greenish tinge. The air-oxidised solid is sometimes known as "green rust".

Iron-oxidizing bacteria are chemotrophic bacteria that derive energy by oxidizing dissolved iron. They are known to grow and proliferate in waters containing iron concentrations as low as 0.1 mg/L. However, at least 0.3 ppm of dissolved oxygen is needed to carry out the oxidation.

Inceptisols are a soil order in USDA soil taxonomy. They form quickly through alteration of parent material. They are more developed than Entisols. They have no accumulation of clays, iron oxide, aluminium oxide or organic matter. They have an ochric or umbric horizon and a cambic subsurface horizon.

Ferrihydrite (Fh) is a widespread hydrous ferric oxyhydroxide mineral at the Earth's surface, and a likely constituent in extraterrestrial materials. It forms in several types of environments, from freshwater to marine systems, aquifers to hydrothermal hot springs and scales, soils, and areas affected by mining. It can be precipitated directly from oxygenated iron-rich aqueous solutions, or by bacteria either as a result of a metabolic activity or passive sorption of dissolved iron followed by nucleation reactions. Ferrihydrite also occurs in the core of the ferritin protein from many living organisms, for the purpose of intra-cellular iron storage.

Fougèrite is a relatively recently described naturally occurring green rust mineral. It is the archetype of the fougèrite group in the larger hydrotalcite supergroup of naturally occurring layered double hydroxides. The structure is based on brucite-like layers containing Fe2+ and Fe3+ cations, O2− and OH− anions, with loosely bound [CO3]2− groups and H2O molecules between the layers. Fougèrite crystallizes in trigonal system. The ideal formula for fougèrite is [Fe2+4Fe3+2(OH)12][CO3]·3H2O. Higher degrees of oxidation produce the other members of the fougèrite group, namely trébeurdenite, [Fe2+2Fe3+4O2(OH)10][CO3]·3H2O and mössbauerite, [Fe3+6O4(OH)8][CO3]·3H2O.
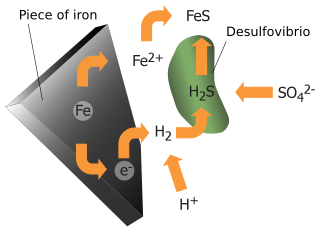
Anaerobic corrosion is a form of metal corrosion occurring in anoxic water. Typically following aerobic corrosion, anaerobic corrosion involves a redox reaction that reduces hydrogen ions and oxidizes a solid metal. This process can occur in either abiotic conditions through a thermodynamically spontaneous reaction or biotic conditions through a process known as bacterial anaerobic corrosion. Along with other forms of corrosion, anaerobic corrosion is significant when considering the safe, permanent storage of chemical waste.

A Planosol in the World Reference Base for Soil Resources is a soil with a light-coloured, coarse-textured, surface horizon that shows signs of periodic water stagnation and abruptly overlies a dense, slowly permeable subsoil with significantly more clay than the surface horizon. In the US Soil Classification of 1938 used the name Planosols, whereas its successor, the USDA soil taxonomy, includes most Planosols in the Great Groups Albaqualfs, Albaquults and Argialbolls.

A Stagnosol in the World Reference Base for Soil Resources (WRB) is soil with strong mottling of the soil profile due to redox processes caused by stagnating surface water.

A redox gradient is a series of reduction-oxidation (redox) reactions sorted according to redox potential. The redox ladder displays the order in which redox reactions occur based on the free energy gained from redox pairs. These redox gradients form both spatially and temporally as a result of differences in microbial processes, chemical composition of the environment, and oxidative potential. Common environments where redox gradients exist are coastal marshes, lakes, contaminant plumes, and soils.

The Schikorr reaction formally describes the conversion of the iron(II) hydroxide (Fe(OH)2) into iron(II,III) oxide (Fe3O4). This transformation reaction was first studied by Gerhard Schikorr. The global reaction follows:

Green rust is a generic name for various green crystalline chemical compounds containing iron(II) and iron(III) cations, the hydroxide (OH−
) anion, and another anion such as carbonate (CO2−
3), chloride (Cl−
), or sulfate (SO2−
4), in a layered double hydroxide (LDH) structure. The most studied varieties are the following:

Nitisol, in the World Reference Base for Soil Resources (WRB), is a deep, red, well-drained soil with a clay content of at least 30% and a polyhedral structure or a blocky structure, breaking into a polyhedral or a flat-edged structure. The soil aggregates show pressure faces. Nitisols correlate with the kandic alfisols, ultisols and inceptisols of the USDA soil taxonomy.
The Polish Soil Classification is a soil classification system used to describe, classify and organize the knowledge about soils in Poland.



















