Related Research Articles

Saliva is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is around 99% water, plus electrolytes, mucus, white blood cells, epithelial cells, enzymes, antimicrobial agents.

Renin, also known as an angiotensinogenase, is an aspartic protease protein and enzyme secreted by the kidneys that participates in the body's renin–angiotensin–aldosterone system (RAAS)—also known as the renin–angiotensin–aldosterone axis—that increases the volume of extracellular fluid and causes arterial vasoconstriction. Thus, it increases the body's mean arterial blood pressure.
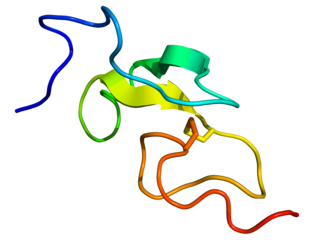
Epidermal growth factor (EGF) is a protein that stimulates cell growth and differentiation by binding to its receptor, EGFR. Human EGF is 6-kDa and has 53 amino acid residues and three intramolecular disulfide bonds.
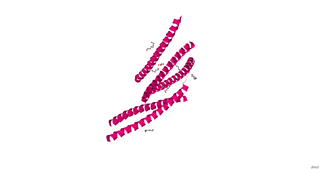
cGMP-dependent protein kinase or protein kinase G (PKG) is a serine/threonine-specific protein kinase that is activated by cGMP. It phosphorylates a number of biologically important targets and is implicated in the regulation of smooth muscle relaxation, platelet function, sperm metabolism, cell division, and nucleic acid synthesis.
Kallikreins are a subgroup of serine proteases, enzymes capable of cleaving peptide bonds in proteins. In humans, plasma kallikrein has no known paralogue, while tissue kallikrein-related peptidases (KLKs) encode a family of fifteen closely related serine proteases. These genes are localised to chromosome 19q13, forming the largest contiguous cluster of proteases within the human genome. Kallikreins are responsible for the coordination of various physiological functions including blood pressure, semen liquefaction and skin desquamation.
Kininogens are precursor proteins for kinins, biologically active polypeptides involved in blood coagulation, vasodilation, smooth muscle contraction, inflammatory regulation, and the regulation of the cardiovascular and renal systems.
Tissue kallikrein is an enzyme. This enzyme catalyses the following chemical reaction

A disintegrin and metalloprotease 17 (ADAM17), also called TACE, is a 70-kDa enzyme that belongs to the ADAM protein family of disintegrins and metalloproteases.

Batroxobin, also known as reptilase, is a snake venom enzyme with Venombin A activity produced by Bothrops atrox and Bothrops moojeni, venomous species of pit viper found east of the Andes in South America. It is a hemotoxin which acts as a serine protease similarly to thrombin, and has been the subject of many medical studies as a replacement of thrombin. Different enzymes, isolated from different species of Bothrops, have been called batroxobin, but unless stated otherwise, this article covers the batroxobin produced by B. moojeni, as this is the most studied variety.

Peptidyl-prolyl cis-trans isomerase FKBP1A is an enzyme that in humans is encoded by the FKBP1A gene. It is also commonly referred to as FKBP-12 or FKBP12 and is a member of a family of FK506-binding proteins (FKBPs).

Cathepsin D is a protein that in humans is encoded by the CTSD gene. This gene encodes a lysosomal aspartyl protease composed of a protein dimer of disulfide-linked heavy and light chains, both produced from a single protein precursor. Cathepsin D is an aspartic endo-protease that is ubiquitously distributed in lysosomes. The main function of cathepsin D is to degrade proteins and activate precursors of bioactive proteins in pre-lysosomal compartments. This proteinase, which is a member of the peptidase A1 family, has a specificity similar to but narrower than that of pepsin A. Transcription of the CTSD gene is initiated from several sites, including one that is a start site for an estrogen-regulated transcript. Mutations in this gene are involved in the pathogenesis of several diseases, including breast cancer and possibly Alzheimer disease. Homozygous deletion of the CTSD gene leads to early lethality in the postnatal phase. Deficiency of CTSD gene has been reported an underlying cause of neuronal ceroid lipofuscinosis (NCL).

Aldo-keto reductase family 1, member B1 (AKR1B1), also known as aldose reductase, is an enzyme that is encoded by the AKR1B1 gene in humans. It is a reduced nicotinamide-adenine dinucleotide phosphate (NADPH)-dependent enzyme catalyzing the reduction of various aldehydes and ketones to the corresponding alcohol. The involvement of AKR1B1 in oxidative stress diseases, cell signal transduction, and cell proliferation process endows AKR1B1 with potential as a therapeutic target.

Kallikrein-6 is a protein that in humans is encoded by the KLK6 gene. Kallikrein-6 is also referred to as neurosin, protease M, hK6, or zyme. It is a 223 amino acid sequence, derived from its 244 original form, which contains a 16 residue presignal and 5 residue activation peptide.

Kallikrein-related peptidase 4 is a protein which in humans is encoded by the KLK4 gene.

Carbonic anhydrase 6 is an enzyme that in humans is encoded by the CA6 gene. It is also called 'gustin' because of its presence in saliva, and lower-than-normal levels of salivary zinc in individuals with hypogeusia.
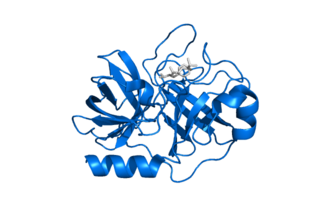
Kallikrein-related peptidase 7 (KLK7) is a serine protease that in humans is encoded by the KLK7 gene. KLK7 was initially purified from the epidermis and characterised as stratum corneum chymotryptic enzyme (SCCE). It was later identified as the seventh member of the human kallikrein family, which includes fifteen homologous serine proteases located on chromosome 19 (19q13).

Kallikrein-14 is a protein that in humans is encoded by the KLK14 gene.

Alpha-2,8-sialyltransferase 8B is an enzyme that in humans is encoded by the ST8SIA2 gene.

Kallikrein-related peptidase 9 also known as KLK9 is an enzyme which in humans is encoded by the KLK9 gene.
Gamma-renin is an enzyme. This enzyme catalyses the following chemical reaction
References
- ↑ Nakayama K, Kim WS, Nakagawa T, Nagahama M, Murakami K (December 1990). "Substrate specificity of prorenin converting enzyme of mouse submandibular gland. Analysis using site-directed mutagenesis". The Journal of Biological Chemistry. 265 (34): 21027–31. PMID 2250008.
- ↑ Kim WS, Hatsuzawa K, Ishizuka Y, Hashiba K, Murakami K, Nakayama K (April 1990). "A processing enzyme for prorenin in mouse submandibular gland. Purification and characterization". The Journal of Biological Chemistry. 265 (11): 5930–3. PMID 2180937.
- ↑ Kikkawa Y, Yamanaka N, Tada J, Kanamori N, Tsumura K, Hosoi K (January 1998). "Prorenin processing and restricted endoproteolysis by mouse tissue kallikrein family enzymes (mK1, mK9, mK13, and mK22)". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1382 (1): 55–64. doi:10.1016/s0167-4838(97)00144-1. PMID 9507064.
- ↑ Yao C, Karabasil MR, Purwanti N, Li X, Akamatsu T, Kanamori N, Hosoi K (March 2006). "Tissue kallikrein mK13 is a candidate processing enzyme for the precursor of interleukin-1beta in the submandibular gland of mice". The Journal of Biological Chemistry. 281 (12): 7968–76. doi: 10.1074/jbc.m507705200 . PMID 16423834.