
In biochemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology. Protein phosphorylation often activates many enzymes.
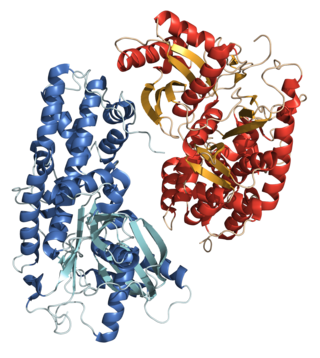
A hexokinase is an enzyme that phosphorylates hexoses, forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexokinase possesses the ability to transfer an inorganic phosphate group from ATP to a substrate.

Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism.

The lactose operon is an operon required for the transport and metabolism of lactose in E. coli and many other enteric bacteria. Although glucose is the preferred carbon source for most bacteria, the lac operon allows for the effective digestion of lactose when glucose is not available through the activity of beta-galactosidase. Gene regulation of the lac operon was the first genetic regulatory mechanism to be understood clearly, so it has become a foremost example of prokaryotic gene regulation. It is often discussed in introductory molecular and cellular biology classes for this reason. This lactose metabolism system was used by François Jacob and Jacques Monod to determine how a biological cell knows which enzyme to synthesize. Their work on the lac operon won them the Nobel Prize in Physiology in 1965.
PEP group translocation, also known as the phosphotransferase system or PTS, is a distinct method used by bacteria for sugar uptake where the source of energy is from phosphoenolpyruvate (PEP). It is known to be a multicomponent system that always involves enzymes of the plasma membrane and those in the cytoplasm.

Phosphoenolpyruvate is the ester derived from the enol of pyruvate and phosphate. It exists as an anion. PEP is an important intermediate in biochemistry. It has the highest-energy phosphate bond found in organisms, and is involved in glycolysis and gluconeogenesis. In plants, it is also involved in the biosynthesis of various aromatic compounds, and in carbon fixation; in bacteria, it is also used as the source of energy for the phosphotransferase system.
The galactose permease or GalP found in Escherichia coli is an integral membrane protein involved in the transport of monosaccharides, primarily hexoses, for utilization by E. coli in glycolysis and other metabolic and catabolic pathways (3,4). It is a member of the Major Facilitator Super Family (MFS) and is homologue of the human GLUT1 transporter (4). Below you will find descriptions of the structure, specificity, effects on homeostasis, expression, and regulation of GalP along with examples of several of its homologues.
UTP—glucose-1-phosphate uridylyltransferase also known as glucose-1-phosphate uridylyltransferase is an enzyme involved in carbohydrate metabolism. It synthesizes UDP-glucose from glucose-1-phosphate and UTP; i.e.,

Histidine kinases (HK) are multifunctional, and in non-animal kingdoms, typically transmembrane, proteins of the transferase class of enzymes that play a role in signal transduction across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase, phosphotransfer, and phosphatase activity. HKs can act as cellular receptors for signaling molecules in a way analogous to tyrosine kinase receptors (RTK). Multifunctional receptor molecules such as HKs and RTKs typically have portions on the outside of the cell that bind to hormone- or growth factor-like molecules, portions that span the cell membrane, and portions within the cell that contain the enzymatic activity. In addition to kinase activity, the intracellular domains typically have regions that bind to a secondary effector molecule or complex of molecules that further propagate signal transduction within the cell. Distinct from other classes of protein kinases, HKs are usually parts of a two-component signal transduction mechanisms in which HK transfers a phosphate group from ATP to a histidine residue within the kinase, and then to an aspartate residue on the receiver domain of a response regulator protein. More recently, the widespread existence of protein histidine phosphorylation distinct from that of two-component histidine kinases has been recognised in human cells. In marked contrast to Ser, Thr and Tyr phosphorylation, the analysis of phosphorylated Histidine using standard biochemical and mass spectrometric approaches is much more challenging, and special procedures and separation techniques are required for their preservation alongside classical Ser, Thr and Tyr phosphorylation on proteins isolated from human cells.
In enzymology, a phosphoenolpyruvate-protein phosphotransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-histidine pros-kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-histidine tele-kinase is an enzyme that catalyzes the chemical reaction
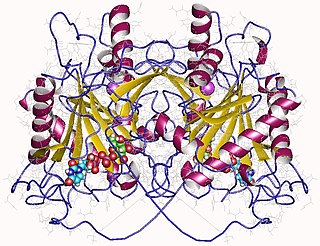
In enzymology, an UDP-glucose—hexose-1-phosphate uridylyltransferase is an enzyme that catalyzes the chemical reaction

Histidine-containing phosphocarrier protein (HPr) is a small cytoplasmic protein that is a component of the phosphoenolpyruvate-dependent sugar phosphotransferase system (PTS).

The bacterial phosphoenolpyruvate: sugar phosphotransferase system (PTS) is a multi-protein system involved in the regulation of a variety of metabolic and transcriptional processes. The PTS catalyzes the phosphorylation of incoming sugar substrates concomitant with their translocation across the cell membrane. The general mechanism of the PTS is the following: a phosphoryl group from phosphoenolpyruvate (PEP) is transferred to enzyme-I (EI) of PTS which in turn transfers it to a phosphoryl carrier protein (HPr). Phospho-HPr then transfers the phosphoryl group to a sugar-specific permease which consists of at least three structurally distinct domains which can either be fused together in a single polypeptide chain or exist as two or three interactive chains, formerly called enzymes II (EII) and III (EIII). The IIC domain catalyzes the transfer of a phosphoryl group from IIB to the sugar substrate.
Thermotoga naphthophila is a hyperthermophilic, anaerobic, non-spore-forming, rod-shaped fermentative heterotroph, with type strain RKU-10T.
The phosphotransferases system (PTS-GFL) superfamily is a superfamily of phosphotransferase enzymes that facilitate the transport of glucose, glucitol (G), fructose (F) and lactose (L). Classification has been established through phylogenic analysis and bioinformatics.
The PTSGlucose-Glucoside (Glc) family includes porters specific for glucose, glucosamine, N-acetylglucosamine and a large variety of α- and β-glucosides, and is part of the PTS-GFL superfamily.
The PTS Lactose-N,N’-Diacetylchitobiose (Lac) Family includes several sequenced lactose porters of Gram-positive bacteria, as well as the Escherichia coli and Borrelia burgdorferi N,N'-diacetylchitobiose (Chb) porters. It is part of the PTS-GFL superfamily. The former can transport aromatic β-glucosides and cellobiose, as well as Chb. However, only Chb induces expression of the chb operon.
Permease of phosphotransferase system is a superfamily of phosphotransferase enzymes that facilitate the transport of L-ascorbate (A) and galactitol (G). Classification has been established through phylogenic analysis and bioinformatics.










