
A retrovirus is a type of virus that inserts a DNA copy of its RNA genome into the DNA of a host cell that it invades, thus changing the genome of that cell. Once inside the host cell's cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backwards). The new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus.

Rubella virus (RuV) is the pathogenic agent of the disease rubella, transmitted only between humans via the respiratory route, and is the main cause of congenital rubella syndrome when infection occurs during the first weeks of pregnancy.

Gammaretrovirus is a genus in the Retroviridae family. Example species are the murine leukemia virus and the feline leukemia virus. They cause various sarcomas, leukemias and immune deficiencies in mammals, reptiles and birds.
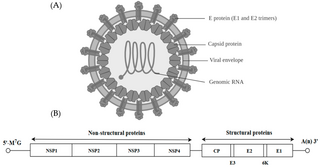
Alphavirus is a genus of RNA viruses, the sole genus in the Togaviridae family. Alphaviruses belong to group IV of the Baltimore classification of viruses, with a positive-sense, single-stranded RNA genome. There are 32 alphaviruses, which infect various vertebrates such as humans, rodents, fish, birds, and larger mammals such as horses, as well as invertebrates. Alphaviruses that could infect both vertebrates and arthropods are referred dual-host alphaviruses, while insect-specific alphaviruses such as Eilat virus and Yada yada virus are restricted to their competent arthropod vector. Transmission between species and individuals occurs mainly via mosquitoes, making the alphaviruses a member of the collection of arboviruses – or arthropod-borne viruses. Alphavirus particles are enveloped, have a 70 nm diameter, tend to be spherical, and have a 40 nm isometric nucleocapsid.
Rous sarcoma virus (RSV) is a retrovirus and is the first oncovirus to have been described. It causes sarcoma in chickens.
The murine leukemia viruses are retroviruses named for their ability to cause cancer in murine (mouse) hosts. Some MLVs may infect other vertebrates. MLVs include both exogenous and endogenous viruses. Replicating MLVs have a positive sense, single-stranded RNA (ssRNA) genome that replicates through a DNA intermediate via the process of reverse transcription.
Group-specific antigen, or gag, is the polyprotein that contains the core structural proteins of an Ortervirus. It was named as such because scientists used to believe it was antigenic. Now it is known that it makes up the inner shell, not the envelope exposed outside. It makes up all the structural units of viral conformation and provides supportive framework for mature virion.
The gag-onc fusion protein is a general term for a fusion protein formed from a group-specific antigen ('gag') gene and that of an oncogene ('onc'), a gene that plays a role in the development of a cancer. The name is also written as Gag-v-Onc, with "v" indicating that the Onc sequence resides in a viral genome. Onc is a generic placeholder for a given specific oncogene, such as C-jun..
Env is a viral gene that encodes the protein forming the viral envelope. The expression of the env gene enables retroviruses to target and attach to specific cell types, and to infiltrate the target cell membrane.
Visna-maedi virus from the genus Lentivirus and subfamily Orthoretrovirinae, is a retrovirus that causes encephalitis and chronic pneumonitis in sheep. It is known as visna when found in the brain, and maedi when infecting the lungs. Lifelong, persistent infections in sheep occur in the lungs, lymph nodes, spleen, joints, central nervous system, and mammary glands; The condition is sometimes known as ovine progressive pneumonia (OPP), particularly in the United States, or Montana sheep disease. White blood cells of the monocyte/macrophage lineage are the main target of the virus.

This family represents the bovine leukaemia virus RNA encapsidation (packaging) signal, which is essential for efficient viral replication.

The retroviral psi packaging element, also known as the Ψ RNA packaging signal, is a cis-acting RNA element identified in the genomes of the retroviruses Human immunodeficiency virus (HIV) and Simian immunodeficiency virus (SIV). It is involved in regulating the essential process of packaging the retroviral RNA genome into the viral capsid during replication. The final virion contains a dimer of two identical unspliced copies of the viral genome.

Glycylpeptide N-tetradecanoyltransferase 1 also known as myristoyl-CoA:protein N-myristoyltransferase 1 (NMT-1) is an enzyme that in humans is encoded by the NMT1 gene. It belongs to the protein N-terminal methyltransferase and glycylpeptide N-tetradecanoyltransferase family of enzymes.
Avian sarcoma leukosis virus (ASLV) is an endogenous retrovirus that infects and can lead to cancer in chickens; experimentally it can infect other species of birds and mammals. ASLV replicates in chicken embryo fibroblasts, the cells that contribute to the formation of connective tissues. Different forms of the disease exist, including lymphoblastic, erythroblastic, and osteopetrotic.
Bovine immunodeficiency virus (BIV) is a retrovirus belonging to the genus Lentivirus. It is similar to the human immunodeficiency virus (HIV) and infects cattle. The cells primarily infected are lymphocytes and monocytes/macrophages.

In molecular biology, barrier-to-autointegration factor (BAF) is a family of essential proteins that is highly conserved in metazoan evolution, and which may act as DNA-bridging proteins. BAF binds directly to double-stranded DNA, to transcription activators, and to inner nuclear membrane proteins, including lamin A filaments that anchor nuclear pore complexes in place, and nuclear LEM-domain proteins that bind to laminin filaments and chromatin. New findings suggest that BAF has structural roles in nuclear assembly and chromatin organization, represses gene expression and might interlink chromatin structure, nuclear architecture and gene regulation in metazoans.
Mason-Pfizer monkey virus (M-PMV), formerly Simian retrovirus (SRV), is a species of retroviruses that usually infect and cause a fatal immune deficiency in Asian macaques. The ssRNA virus appears sporadically in mammary carcinoma of captive macaques at breeding facilities which expected as the natural host, but the prevalence of this virus in feral macaques remains unknown. M-PMV was transmitted naturally by virus-containing body fluids, via biting, scratching, grooming, and fighting. Cross contaminated instruments or equipment (fomite) can also spread this virus among animals.
Bovine foamy virus (BFV) is a ss(+)RNA retrovirus that belongs to the genus spumaviridae. Spumaviruses differ from the other six members of family retroviridae, both structurally and in pathogenic nature. Spumaviruses derive their name from spuma the latin for "foam". The 'foam' aspect of 'foamy virus' comes from syncytium formation and the rapid vacuolization of infected cells, creating a 'foamy' appearance.

Ortervirales is an order that contains all accepted species of single-stranded RNA viruses that replicate through a DNA intermediate and all accepted species of double-stranded DNA viruses that replicate through an RNA intermediate . The name is derived from the reverse of retro.
Rio Negro virus is an alphavirus that was first isolated in Argentina in 1980. The virus was first called Ag80-663 but was renamed to Rio Negro virus in 2005. It is a former member of the Venezuelan equine encephalitis complex (VEEC), which are a group of alphaviruses in the Americas that have the potential to emerge and cause disease. Río Negro virus was recently reclassified as a distinct species. Closely related viruses include Mucambo virus and Everglades virus.












