Related Research Articles

Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion.

Bradykinin (BK) (Greek brady-, slow; -kinin, kīn(eîn) to move) is a peptide that promotes inflammation. It causes arterioles to dilate (enlarge) via the release of prostacyclin, nitric oxide, and endothelium-derived hyperpolarizing factor and makes veins constrict, via prostaglandin F2, thereby leading to leakage into capillary beds, due to the increased pressure in the capillaries. Bradykinin consists of nine amino acids, and is a physiologically and pharmacologically active peptide of the kinin group of proteins.

Serine proteases are enzymes that cleave peptide bonds in proteins. Serine serves as the nucleophilic amino acid at the (enzyme's) active site. They are found ubiquitously in both eukaryotes and prokaryotes. Serine proteases fall into two broad categories based on their structure: chymotrypsin-like (trypsin-like) or subtilisin-like.

The Viperidae (vipers) are a family of snakes found in most parts of the world, except for Antarctica, Australia, Hawaii, Madagascar, New Zealand, and various other isolated islands. They are venomous and have long, hinged fangs that permit deep penetration and injection of their venom. Three subfamilies are currently recognized. They are also known as viperids. The name "viper" is derived from the Latin word vipera, -ae, also meaning viper, possibly from vivus ("living") and parere, referring to the trait viviparity common in vipers like most of the species of Boidae.

A snakebite is an injury caused by the bite of a snake, especially a venomous snake. A common sign of a bite from a venomous snake is the presence of two puncture wounds from the animal's fangs. Sometimes venom injection from the bite may occur. This may result in redness, swelling, and severe pain at the area, which may take up to an hour to appear. Vomiting, blurred vision, tingling of the limbs, and sweating may result. Most bites are on the hands, arms, or legs. Fear following a bite is common with symptoms of a racing heart and feeling faint. The venom may cause bleeding, kidney failure, a severe allergic reaction, tissue death around the bite, or breathing problems. Bites may result in the loss of a limb or other chronic problems or even death.

Snake venom is a highly toxic saliva containing zootoxins that facilitates in the immobilization and digestion of prey. This also provides defense against threats. Snake venom is injected by unique fangs during a bite, whereas some species are also able to spit venom.

Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.

The enzyme phospholipase A2 (EC 3.1.1.4, PLA2, systematic name phosphatidylcholine 2-acylhydrolase) catalyse the cleavage of fatty acids in position 2 of phospholipids, hydrolyzing the bond between the second fatty acid “tail” and the glycerol molecule:

Dilute Russell's viper venom time (dRVVT) is a laboratory test often used for detection of lupus anticoagulant (LA).
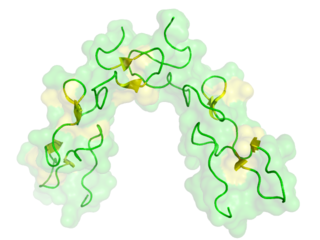
Disintegrins are a family of small proteins from viper venoms that function as potent inhibitors of both platelet aggregation and integrin-dependent cell adhesion.

The Indian cobra, also known commonly as the spectacled cobra, Asian cobra, or binocellate cobra, is a species of cobra, a venomous snake in the family Elapidae. The species is native to the Indian subcontinent, and is a member of the "big four" species that are responsible for the most snakebite cases in India.
Kallikreins are a subgroup of serine proteases, enzymes capable of cleaving peptide bonds in proteins. In humans, plasma kallikrein has no known paralogue, while tissue kallikrein-related peptidases (KLKs) encode a family of fifteen closely related serine proteases. These genes are localised to chromosome 19q13, forming the largest contiguous cluster of proteases within the human genome. Kallikreins are responsible for the coordination of various physiological functions including blood pressure, semen liquefaction and skin desquamation.

Sphingomyelin phosphodiesterase is a hydrolase enzyme that is involved in sphingolipid metabolism reactions. SMase is a member of the DNase I superfamily of enzymes and is responsible for breaking sphingomyelin (SM) down into phosphocholine and ceramide. The activation of SMase has been suggested as a major route for the production of ceramide in response to cellular stresses.
Ancrod is a defibrinogenating agent derived from the venom of the Malayan pit viper. Defibrinogenating blood produces an anticoagulant effect. Ancrod is not approved or marketed in any country. It is a thrombin-like serine protease.

Batroxobin, also known as reptilase, is a snake venom enzyme with Venombin A activity produced by Bothrops atrox and Bothrops moojeni, venomous species of pit viper found east of the Andes in South America. It is a hemotoxin which acts as a serine protease similarly to thrombin, and has been the subject of many medical studies as a replacement of thrombin. Different enzymes, isolated from different species of Bothrops, have been called batroxobin, but unless stated otherwise, this article covers the batroxobin produced by B. moojeni, as this is the most studied variety.
Venom-induced consumption coagulopathy (VICC) is a medical condition caused by the effects of some snake and caterpillar venoms on the blood. Important coagulation factors are activated by the specific serine proteases in the venom and as they become exhausted, coagulopathy develops. Symptoms are consistent with uncontrolled bleeding. Diagnosis is made using blood tests that assess clotting ability along with recent history of envenomation. Treatment generally involves pressure dressing, confirmatory blood testing, and antivenom administration.

In enzymology, an L-amino acid oxidase (LAAO) (EC 1.4.3.2) is an enzyme that catalyzes the chemical reaction
Cerastocytin is a thrombin-like serine protease in snake venom.
Scutelarin is an enzyme. This enzyme catalyses the following chemical reaction
Russellysin is an enzyme. This enzyme catalyses the following chemical reaction
References
- ↑ Kisiel W, Canfield WM (1981). Snake venom proteases that activate blood-coagulation factor V. Methods in Enzymology. Vol. 80 Pt C. pp. 275–85. doi:10.1016/s0076-6879(81)80024-9. PMID 7043192.
- ↑ Tokunaga F, Nagasawa K, Tamura S, Miyata T, Iwanaga S, Kisiel W (November 1988). "The factor V-activating enzyme (RVV-V) from Russell's viper venom. Identification of isoproteins RVV-V alpha, -V beta, and -V gamma and their complete amino acid sequences". The Journal of Biological Chemistry. 263 (33): 17471–81. PMID 3053712.