isoleucine is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, an α-carboxylic acid group, and a hydrocarbon side chain with a branch. It is classified as a non-polar, uncharged, branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it. Essential amino acids are necessary in the human diet. In plants isoleucine can be synthesized from threonine and methionine. In plants and bacteria, isoleucine is synthesized from a pyruvate employing leucine biosynthesis enzymes. It is encoded by the codons AUU, AUC, and AUA.

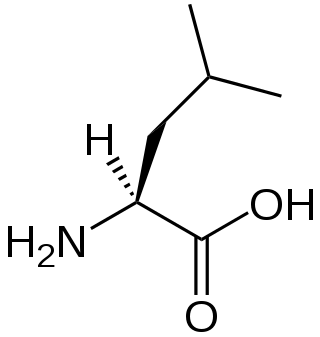
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it; it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG. Leucine is named after the Greek word for "white": λευκός (leukós, "white"), after its common appearance as a white powder, a property it shares with many other amino acids.

Maple syrup urine disease (MSUD) is a rare, inherited metabolic disorder that affects the body's ability to metabolize amino acids due to a deficiency in the activity of the branched-chain alpha-ketoacid dehydrogenase (BCKAD) complex. It particularly affects the metabolism of amino acids—leucine, isoleucine, and valine. With MSUD, the body is not able to properly break down these amino acids, therefore leading to the amino acids to build up in urine and become toxic. The condition gets its name from the distinctive sweet odor of affected infants' urine and earwax due to the buildup of these amino acids.
Bodybuilding supplements are dietary supplements commonly used by those involved in bodybuilding, weightlifting, mixed martial arts, and athletics for the purpose of facilitating an increase in lean body mass. Bodybuilding supplements may contain ingredients that are advertised to increase a person's muscle, body weight, athletic performance, and decrease a person's percent body fat for desired muscle definition. Among the most widely used are high protein drinks, pre-workout blends, branched-chain amino acids (BCAA), glutamine, arginine, essential fatty acids, creatine, HMB, whey protein, ZMA, and weight loss products. Supplements are sold either as single ingredient preparations or in the form of "stacks" – proprietary blends of various supplements marketed as offering synergistic advantages.

β-Hydroxy β-methylbutyric acid (HMB), otherwise known as its conjugate base, β-hydroxyβ-methylbutyrate, is a naturally produced substance in humans that is used as a dietary supplement and as an ingredient in certain medical foods that are intended to promote wound healing and provide nutritional support for people with muscle wasting due to cancer or HIV/AIDS. In healthy adults, supplementation with HMB has been shown to increase exercise-induced gains in muscle size, muscle strength, and lean body mass, reduce skeletal muscle damage from exercise, improve aerobic exercise performance, and expedite recovery from exercise. Medical reviews and meta-analyses indicate that HMB supplementation also helps to preserve or increase lean body mass and muscle strength in individuals experiencing age-related muscle loss. HMB produces these effects in part by stimulating the production of proteins and inhibiting the breakdown of proteins in muscle tissue. No adverse effects from long-term use as a dietary supplement in adults have been found.
A branched-chain amino acid (BCAA) is an amino acid having an aliphatic side-chain with a branch. Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine, and valine. Non-proteinogenic BCAAs include 2-aminoisobutyric acid and alloisoleucine.
The branched-chain α-ketoacid dehydrogenase complex is a multi-subunit complex of enzymes that is found on the mitochondrial inner membrane. This enzyme complex catalyzes the oxidative decarboxylation of branched, short-chain alpha-ketoacids. BCKDC is a member of the mitochondrial α-ketoacid dehydrogenase complex family, which also includes pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase, key enzymes that function in the Krebs cycle.

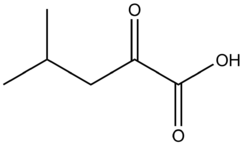
Isovaleric acid, also known as 3-methylbutanoic acid or β-methylbutyric acid, is a branched-chain alkyl carboxylic acid with the chemical formula (CH3)2CHCH2CO2H. It is classified as a short-chain fatty acid. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. The compound occurs naturally and can be found in many foods, such as cheese, soy milk, and apple juice.

Proteasome inhibitors are drugs that block the action of proteasomes. Proteasomes are large proteins complexes that are used to break down other proteins. These inhibitors are being studied for the treatment of cancer. Drugs such as bortezomib, carfilzomib, and ixazomib are already approved for use in treating multiple myeloma and mantle cell lymphoma. They also work as immunosuppressants and inhibit bone resorption.

β-Hydroxy β-methylglutaryl-CoA (HMG-CoA), also known as 3-hydroxy-3-methylglutaryl coenzyme A, is an intermediate in the mevalonate and ketogenesis pathways. It is formed from acetyl CoA and acetoacetyl CoA by HMG-CoA synthase. The research of Minor J. Coon and Bimal Kumar Bachhawat in the 1950s at University of Illinois led to its discovery.

Enoyl-CoA hydratase (ECH) or crotonase is an enzyme EC 4.2.1.17 that hydrates the double bond between the second and third carbons on 2-trans/cis-enoyl-CoA:
Methylcrotonyl CoA carboxylase is a biotin-requiring enzyme located in the mitochondria. MCC uses bicarbonate as a carboxyl group source to catalyze the carboxylation of a carbon adjacent to a carbonyl group performing the fourth step in processing leucine, an essential amino acid.

Isovaleryl-coenzyme A, also known as isovaleryl-CoA, is an intermediate in the metabolism of branched-chain amino acids.

3-Methylcrotonyl-CoA is an intermediate in the metabolism of leucine.

3-Methylglutaconyl-CoA (MG-CoA), also known as β-methylglutaconyl-CoA, is an intermediate in the metabolism of leucine. It is metabolized into HMG-CoA.

3-Methylglutaconyl-CoA hydratase, also known as MG-CoA hydratase and AUH, is an enzyme encoded by the AUH gene on chromosome 19. It is a member of the enoyl-CoA hydratase/isomerase superfamily, but it is the only member of that family that is able to bind to RNA. Not only does it bind to RNA, AUH has also been observed to be involved in the metabolic enzymatic activity, making it a dual-role protein. Mutations of this gene have been found to cause a disease called 3-Methylglutaconic Acuduria Type 1.

In enzymology, an isovaleryl-CoA dehydrogenase is an enzyme that catalyzes the chemical reaction

A 2-oxoisovalerate dehydrogenase subunit alpha, mitochondrial is an enzyme that in humans is encoded by the BCKDHA gene.

β-Hydroxy β-methylbutyryl-coenzyme A (HMB-CoA), also known as 3-hydroxyisovaleryl-CoA, is a metabolite of L-leucine that is produced in the human body. Its immediate precursors are β-hydroxy β-methylbutyric acid (HMB) and β-methylcrotonoyl-CoA (MC-CoA). It can be metabolized into HMB, MC-CoA, and HMG-CoA in humans.
Juven is a medical food that is manufactured by Abbott Laboratories and used to provide nutritional support under the care of a physician in individuals with muscle wasting due to AIDS or cancer, to promote wound healing following surgery or injury, or when otherwise recommended by a medical professional. It is a powdered nutritional supplement that contains 3 grams of calcium β-hydroxy β-methylbutyrate, 14 grams of L-arginine, and 14 grams of L-glutamine per two daily servings.